Choroidal Neovascularization Market Summary
- Choroidal neovascularization is the growth of abnormal blood vessels beneath the retina that leak fluid or blood, causing blurred or distorted central vision. Commonly linked to neovascular (wet) age-related macular degeneration (nAMD), myopia, or inflammation, choroidal neovascularization can severely affect daily activities and, if untreated, may lead to permanent vision loss.
- Our secondary research indicates that in the US, 0.017% of the population is affected by myopic choroidal neovascularization, highlighting its role as a notable cause of vision loss despite its low prevalence.
- In Germany, pathological myopia affects about 1–3% of the population, with 5–11% developing choroidal neovascularization. Though relatively uncommon, this complication is a leading cause of central vision loss and significantly impacts quality of life.
- VABYSMO (faricimab-svoa), developed by Genentech, and BEOVU (brolucizumab-dbll), developed by Novartis, are both approved by the US Food and Drug Administration (FDA) for the treatment of choroidal neovascularization associated with neovascular (wet) age-related macular degeneration. These approvals represent major advances in retinal therapy, offering targeted anti-VEGF options that help preserve vision and reduce treatment burden for patients.
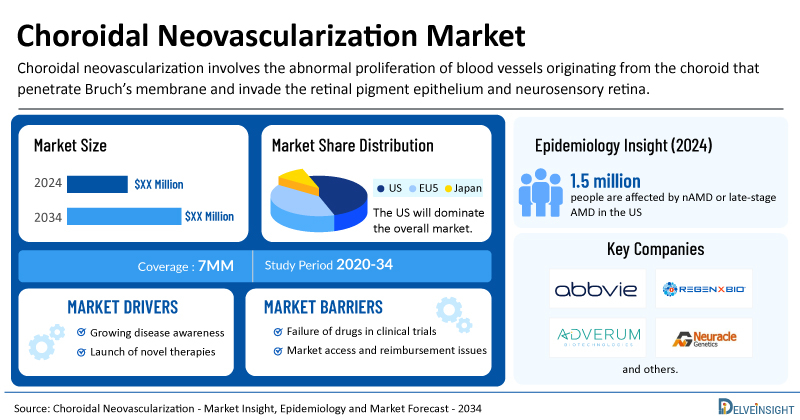
- Emerging gene therapies such as surabgene lomparvovec (ABBV-RGX-314) by AbbVie/REGENXBIO, NG101 by Neuracle Genetics, and Ixoberogene soroparvovec by Adverum Biotechnologies are driving a shift toward durable, one-time treatments for choroidal neovascularization, aiming to address root causes, reduce treatment burden, and enable lasting vision preservation.
DelveInsight’s comprehensive report titled “Choroidal Neovascularization — Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of choroidal neovascularization. The report presents historical and projected epidemiological data covering total prevalent cases of pathological myopia, total prevalent cases of AMD, total diagnosed prevalent cases of choroidal neovascularization, gender-specific diagnosed prevalent cases of choroidal neovascularization, age-specific diagnosed prevalent cases of choroidal neovascularization, and treated cases of choroidal neovascularization. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing treatment practices and unmet medical requirements in choroidal neovascularization. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan |
|
Choroidal Neovascularization Epidemiology |
|
|
Choroidal Neovascularization Market |
|
|
Market Analysis |
|
|
Choroidal Neovascularization Market players |
|
|
Future opportunity |
Future opportunities in the management of choroidal neovascularization lie in the advancement and integration of both approved and emerging therapies that are transforming the treatment landscape. With VABYSMO (faricimab-svoa) by Genentech and BEOVU (brolucizumab-dbll) by Novartis leading current standards through dual-target and extended-duration mechanisms, the field is now shifting toward durable, one-time interventions. Promising investigational candidates such as surabgene lomparvovec (ABBV-RGX-314), NG101, and Ixoberogene soroparvovec represent the next generation of solutions aimed at long-term disease control and reduced treatment burden. Together, these innovations mark a move toward mechanism-driven, personalized, and sustained therapeutic approaches that could redefine outcomes and significantly enhance quality of life for patients with choroidal neovascularization. |
Key Factors Driving the Growth of the Choroidal Neovascularization Market
Rising prevalence of AMD and aging populations
The global increase in age-related macular degeneration (the leading cause of CNV) and the expanding geriatric population are the primary demand drivers, with more patients, more diagnoses, and long-term treatment needs.
Improved diagnostics and screening
The wider adoption of high-resolution retinal imaging (OCT, OCT angiography) and remote screening programs increases early detection rates and referrals to treatment, thereby enlarging the treated population. This also enables treat-and-extend protocols that improve clinic throughput and adherence.
Launch of emerging choroidal neovascularization gene therapies
The anticipated launch of emerging choroidal neovascularization gene therapies, such as surabgene lomparvovec (ABBV-RGX-314) by AbbVie/REGENXBIO, NG101 by Neuracle Genetics, and Ixoberogene soroparvovec by Adverum Biotechnologies, among others, is expected to change the market dynamics.
Choroidal Neovascularization Overview
Choroidal neovascularization is characterized by the abnormal growth of blood vessels from the choroid that extend through Bruch’s membrane into the retinal pigment epithelium and neurosensory retina, resulting in fluid leakage, bleeding, and scarring that can lead to irreversible central vision loss. It most commonly develops secondary to nAMD and pathological myopia, both of which are major causes of vision impairment worldwide. Patients typically experience blurred or distorted central vision, metamorphopsia, and dark or blank spots in their visual field. The underlying mechanism involves increased expression of vascular endothelial growth factor (VEGF) driven by hypoxia, inflammation, and oxidative stress, which stimulate abnormal angiogenesis and vascular permeability. Diagnosis primarily relies on imaging modalities such as optical coherence tomography (OCT) and fluorescein angiography to detect subretinal fluid and neovascular membranes. Choroidal neovascularization remains a leading cause of severe central vision loss, particularly among older adults and individuals with high myopia, emphasizing the importance of timely diagnosis and intervention to preserve visual function.
Choroidal Neovascularization Diagnosis and Treatment Overview
Choroidal neovascularization is diagnosed primarily through detailed clinical evaluation and advanced ocular imaging. Diagnosis involves a comprehensive patient history focusing on the onset and progression of visual symptoms such as blurred or distorted central vision, metamorphopsia, or central scotomas. Key diagnostic tools include optical coherence tomography (OCT), which detects subretinal or intraretinal fluid and neovascular membranes, and fluorescein angiography, which visualizes leakage from abnormal choroidal vessels. Indocyanine green angiography may also be used to assess deeper vascular layers, particularly in complex or atypical cases. These imaging techniques together confirm the presence and activity of choroidal neovascularization and help distinguish it from other retinal pathologies.
Treatment for choroidal neovascularization is individualized, with no single therapy universally effective across all patients. Current management primarily focuses on inhibiting abnormal vessel growth, reducing vascular leakage, and preserving vision. The standard of care involves intravitreal anti-VEGF injections, which directly suppress the signaling pathways driving neovascularization. In certain cases, photodynamic therapy or laser photocoagulation may be considered. The treatment regimen and frequency depend on disease activity, visual outcomes, and patient response. Early detection and timely therapy are critical to prevent irreversible retinal damage and maintain long-term visual function.
Further details related to disease understanding are provided in the report…
Choroidal Neovascularization Epidemiology
The epidemiology section of the choroidal neovascularization market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the total prevalence rate of choroidal neovascularization in nAMD and pathological myopia, supported by relevant tables and graphs to provide a clear and concise understanding of the data. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
- In the US, around 1.5 million people are affected by nAMD or late-stage AMD, underscoring the major visual and functional burden of choroidal neovascularization.
- In Germany, approximately 3.27 million individuals are affected by AMD, representing a significant public health concern and highlighting the growing need for enhanced screening, timely intervention, and improved access to effective therapies for choroidal neovascularization.
- In France, the prevalence of nAMD is estimated at 1.06%, with female accounting for 64.1% of cases and male 35.9%. The prevalence increases markedly with age, rising from 1.28% in individuals aged ≥55 years to 6.27% in those aged ≥85 years.
- In Japan, the prevalence of nAMD is estimated at 0.52%, while myopic choroidal neovascularization accounts for approximately 11.3% of total cases of high myopia. This distribution reflects the distinct regional pattern of choroidal neovascularization, where high myopia contributes significantly to disease burden, underscoring the importance of early identification and long-term monitoring in at-risk populations.
Choroidal Neovascularization Market Outlook
The choroidal neovascularization therapeutics market is further expected to increase by the major drivers, such as the rising prevalent population, technological advancements, and upcoming therapies in the forecast period (2025–2034).
Choroidal neovascularization is primarily managed through intravitreal anti-VEGF therapy, which targets abnormal blood vessel growth and leakage beneath the retina—the key drivers of vision loss. The most widely used treatments include VABYSMO (faricimab-svoa) by Genentech, the first bispecific antibody targeting both VEGF-A and Ang-2 pathways, and BEOVU (brolucizumab-dbll) by Novartis, designed for sustained efficacy with extended dosing intervals. These agents have demonstrated significant improvements in visual acuity and retinal fluid reduction. Emerging therapies, such as surabgene lomparvovec (ABBV-RGX-314) by AbbVie/REGENXBIO, Ixoberogene soroparvovec by Adverum Biotechnologies, and NG101 by Neuracle Genetics, aim to provide long-term VEGF suppression through one-time administration, potentially reducing treatment burden. Complementary approaches, including laser photocoagulation and photodynamic therapy, may be considered in select cases. Collectively, these advancements are reshaping management by shifting from frequent injections to durable, mechanism-driven therapies that offer sustained vision preservation.
With ongoing research and continued dedication, the future holds promise for even more effective treatments and, ultimately, a potential cure for this challenging condition. According to DelveInsight, the choroidal neovascularization market in the 7MM is expected to change significantly during the forecast period (2025–2034).
Choroidal Neovascularization Drug Chapters
Marketed Drugs
VABYSMO (faricimab-svoa): Genentech/ Chugai Pharmaceutical
VABYSMO (faricimab-svoa), developed by Genentech, is a first-in-class bispecific antibody approved by the US FDA for the treatment of choroidal neovascularization associated with nAMD. It uniquely targets both angiopoietin-2 (Ang-2) and vascular endothelial growth factor A (VEGF-A), addressing two key pathways involved in abnormal blood vessel growth and vascular leakage. By stabilizing retinal vasculature and reducing inflammation, VABYSMO offers durable vision outcomes with extended dosing intervals, representing a significant advancement in the management of choroidal neovascularization.
- In September 2022, the European Commission approved Roche’s VABYSMO (faricimab) for the treatment of nAMD and visual impairment due to DME, marking it as the first bispecific antibody targeting both VEGF-A and Ang-2, developed using Roche’s CrossMab technology.
- In March 2022, Chugai obtained regulatory approval in Japan for VABYSMO (faricimab), the first bispecific antibody in ophthalmology, for the treatment of nAMD and DME, representing a major advancement in retinal disease management.
- In January 2022, the US FDA approved Genentech’s VABYSMO (faricimab-svoa), the first bispecific antibody for the eye, for the treatment of nAMD and DME, marking a major advance with dual Ang-2 and VEGF-A inhibition and flexible dosing options.
BEOVU (brolucizumab-dbll): Novartis
BEOVU (brolucizumab-dbll), developed by Novartis, is an innovative, next-generation anti-VEGF therapy approved for the treatment of choroidal neovascularization associated with nAMD. Designed as a single-chain antibody fragment, it delivers high molar potency with deeper retinal tissue penetration, effectively inhibiting VEGF to reduce vascular leakage and abnormal vessel growth. This mechanism offers sustained control of disease activity with extended dosing intervals, positioning BEOVU as an advanced therapeutic option aimed at improving long-term visual outcomes and reducing treatment burden for patients with choroidal neovascularization.
- In February 2020, Novartis received European Commission approval for BEOVU (brolucizumab-dbll), a next-generation anti-VEGF therapy for nAMD, one of the leading causes of blindness worldwide.
- In October 2019, Novartis received US FDA approval for BEOVU (brolucizumab-dbll) for the treatment of nAMD, offering patients significant vision gains and greater reductions in retinal fluid compared to aflibercept, marking a key advancement in choroidal neovascularization management.
|
Drug |
MoA |
RoA |
Company |
Logo |
|
VABYSMO (faricimab-svoa) |
Dual Ang-2 and VEGF-A inhibition |
Intravitreal injection |
Genentech/ Chugai Pharmaceutical |
|
|
BEOVU (brolucizumab-dbll) |
VEGF-A inhibition |
Intravitreal injection |
Novartis |
|
|
XX |
XX |
XX |
XXX |
|
Note: Detailed marketed therapies assessment will be provided in the final report.
Emerging Drugs
Surabgene Lomparvovec (ABBV-RGX-314): AbbVie/ REGENXBIO
Surabgene lomparvovec (ABBV-RGX-314), developed by AbbVie and REGENXBIO, is an investigational gene therapy for the treatment of choroidal neovascularization associated with nAMD. It utilizes an adeno-associated virus (AAV8) vector to deliver a gene encoding a therapeutic antibody fragment designed to inhibit VEGF production directly within retinal cells. This one-time treatment aims to provide continuous intraocular anti-VEGF expression, reducing the need for frequent injections and offering the potential for long-term control of disease activity and preservation of vision in patients with choroidal neovascularization.
- In September 2021, AbbVie and REGENXBIO partnered to develop surabgene lomparvovec, a one-time gene therapy for nAMD and diabetic retinopathy, currently in pivotal and Phase II trials using subretinal and suprachoroidal delivery approaches.
- Data from the ATMOSPHERE and ASCENT pivotal trials evaluating the safety and efficacy of the subretinal delivery of ABBV-RGX-314 in patients with wet AMD are expected in 2026.
NG101: Neuracle Genetics
NG101, developed by Neuracle Genetics, is an investigational gene therapy being studied for the treatment of choroidal neovascularization associated with nAMD. It is designed to deliver a therapeutic gene to retinal cells to inhibit abnormal blood vessel growth and stabilize retinal structure. By targeting the underlying molecular pathways driving disease progression, NG101 aims to offer a long-lasting, one-time treatment approach that preserves vision and reduces the need for frequent intravitreal injections.
- In November 2024, Neuracle Genetics reported that its investigational gene therapy NG101 received FDA Fast Track Designation (FTD) and completed dosing for Cohort 1, marking a key milestone in its development for the treatment of choroidal neovascularization associated with nAMD.
Ixoberogene soroparvovec: Adverum Biotechnologies
Ixoberogene soroparvovec, developed by Adverum Biotechnologies, is an innovative, one-time gene therapy designed for the treatment of choroidal neovascularization associated with nAMD. Utilizing a proprietary adeno-associated viral (AAV.7m8) vector, it delivers a gene encoding an anti-VEGF therapeutic protein directly to retinal cells, enabling sustained intraocular expression to suppress abnormal blood vessel growth and vascular leakage. This approach aims to provide durable disease control, reduce or eliminate the need for frequent intravitreal injections, and preserve long-term visual function in patients with choroidal neovascularization.
- In September 2025, Adverum Biotechnologies announced the completion of screening for its pivotal Phase III ARTEMIS trial evaluating ixoberogene soroparvovec in patients with choroidal neovascularization associated with nAMD. The company now anticipates full enrollment of at least 284 treatment-naïve and treatment-experienced patients in fourth quarter of 2025, with a data readout expected in first quarter of 2027.
- In November 2024, Adverum Biotechnologies reported positive 52-week LUNA and 4-year OPTIC results for ixoberogene soroparvovec, demonstrating durable efficacy and safety in choroidal neovascularization associated with nAMD.
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
Surabgene lomparvovec (ABBV-RGX-314) |
Anti-VEGF gene therapy |
Subretinal |
AbbVie/ REGENXBIO |
|
II/III |
|
NG101 |
Anti-VEGF expression |
Intravitreal injection |
Neuracle Genetics |
|
I/II |
|
Ixoberogene soroparvovec |
Sustained VEGF inhibition |
Intravitreal injection |
Adverum Biotechnologies |
|
II |
|
XX |
XX |
X |
XXX |
|
XXX |
Note: Detailed marketed/emerging therapies assessment will be provided in the final report.
Choroidal Neovascularization Market Segmentation
DelveInsight’s “Choroidal Neovascularization – Market Insights, Epidemiology, and Market Forecast – 2034” report provides a detailed outlook of the current and future choroidal neovascularization market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Choroidal Neovascularization Market Size by Countries
The choroidal neovascularization market size is assessed separately for various countries, including the US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2024, the United States held a significant share of the overall 7MM (Seven Major Markets) choroidal neovascularization market, primarily attributed to the country’s higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
|
Country-wise Market Size Distribution of Choroidal Neovascularization |
|
|
Choroidal Neovascularization Market Size by Therapies
Choroidal Neovascularization Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034.
|
Market Share Distribution of Choroidal Neovascularization by Therapies in 2034e |
|
|
Note: Detailed market segment assessment will be provided in the final report.
Choroidal Neovascularization Drugs Uptake
This section focuses on the sales uptake of potential choroidal neovascularization drugs that have recently been launched or are anticipated to be launched in the choroidal neovascularization market between 2025 and 2034. It estimates the market penetration of choroidal neovascularization drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the choroidal neovascularization market.
The emerging choroidal neovascularization therapies are analyzed based on various attributes such as efficacy and safety in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the choroidal neovascularization market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Choroidal Neovascularization.
Choroidal Neovascularization Market Access and Reimbursement
DelveInsight’s “Choroidal Neovascularization – Market Insights, Epidemiology, and Market Forecast – 2034” report provides a descriptive overview of the market access and reimbursement scenario of choroidal neovascularization. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current choroidal neovascularization market trends and to fill gaps in secondary findings, we interview KOLs’ and SMEs’ working in the choroidal neovascularization domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or choroidal neovascularization market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the choroidal neovascularization unmet needs.
Choroidal Neovascularization: KOL Insights
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as University of Texas MD Anderson Cancer Center, US; University Medical Center Hamburg-Eppendorf, Germany; PSL Research University, France; University of Campania "Luigi Vanvitelli, Italy; Complutense University, Spain; Liverpool John Moores University, UK; and Keio University School of Medicine, Japan; among others.
As per KOLs from the US, “Choroidal neovascularization remains one of the leading causes of vision loss in aging populations, particularly among patients with nAMD and high myopia. Experts highlight that the evolution of anti-VEGF and emerging gene therapy platforms is transforming long-term disease control by reducing treatment burden and improving durability. However, they also stress the need for earlier detection strategies and real-world data integration to optimize patient outcomes.”
As per KOLs from Germany, “Despite significant progress with biologics and bispecific antibodies, managing choroidal neovascularization continues to pose challenges in clinical practice due to variable response rates and frequent injections. Specialists underline the importance of developing sustained-delivery and gene-based approaches to achieve lasting vascular stabilization. Furthermore, greater emphasis on individualized monitoring and treatment adaptation is viewed as essential to maintain functional vision and patient quality of life.”
As per KOLs from Japan, “The prevalence of choroidal neovascularization, particularly secondary to high myopia, remains a growing concern given the country’s aging and myopia-prone population. Clinicians emphasize that treatment accessibility and adherence are key barriers, especially among elderly patients. Experts advocate for expanding non-invasive and long-acting therapeutic options while promoting screening initiatives to detect disease at earlier, more manageable stages.”
Note: Detailed assessment of KOL Views will be provided in the full report on Choroidal Neovascularization.
Competitive Intelligence Analysis
We conduct a competitive and market intelligence analysis of the choroidal neovascularization. Market, utilizing various Competitive Intelligence tools such as SWOT analysis and market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Choroidal Neovascularization Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for choroidal neovascularization. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging choroidal neovascularization therapies.
Choroidal Neovascularization Report Insights
- Choroidal Neovascularization Patient Population
- Therapeutic Approaches
- Choroidal Neovascularization Pipeline Analysis
- Choroidal Neovascularization Market Size and Trends
- Choroidal Neovascularization Market Opportunities
- Impact of Upcoming Therapies
Choroidal Neovascularization Report Key Strengths
- 10 Years Forecast
- The 7MM Coverage
- Choroidal Neovascularization Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Choroidal Neovascularization Market
- Choroidal Neovascularization Drugs Uptake
Choroidal Neovascularization Report Assessment
- Choroidal Neovascularization Current Treatment Practices
- Unmet Needs
- Choroidal Neovascularization Product Profiles
- Choroidal Neovascularization Market Attractiveness
Key Questions
- How common is choroidal neovascularization?
- What are the key findings of choroidal neovascularization epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for choroidal neovascularization?
- What are the disease risk, burden, and unmet needs of choroidal neovascularization?
- At what CAGR is the choroidal neovascularization market and its epidemiology is expected to grow in the 7MM during the forecast period (2025–2034)?
- How would the unmet needs impact the choroidal neovascularization market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of choroidal neovascularization in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2025–2034)?
- How many companies are currently developing therapies for the treatment of choroidal neovascularization?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the choroidal neovascularization market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of current treatment in the US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the attribute analysis section to provide visibility around leading classes.
- Highlights of Market Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.