Chronic Hepatitis B Market Summary
-
The Chronic Hepatitis B market in the 7MM was valued ~USD 1,603 million in 2025 over the forecast period from 2025 to 2034.
-
The Chronic Hepatitis B market is projected to grow at a CAGR of 12.3% by 2034 in leading countries (US, EU4, UK and Japan)
Factors affecting Chronic Hepatitis B Virus Market Growth
-
Rising Global Prevalence of Hepatitis B
The increasing number of chronic hepatitis B cases worldwide is a primary driver for the market, creating higher demand for diagnostics, antiviral therapies, and disease management solutions.
-
Advancements in Antiviral Therapies
Development of effective antiviral drugs, including nucleos(t)ide analogs and novel therapeutic candidates, improves viral suppression and patient outcomes, fueling market growth.
-
Growing Awareness and Screening Programs
Increasing awareness among healthcare professionals and patients about CHB and its complications, such as liver cirrhosis and hepatocellular carcinoma, drives early diagnosis and treatment adoption.
-
Expansion of Vaccination Programs
Widespread hepatitis B vaccination campaigns help reduce disease spread, but the existing large population of chronic carriers still drives ongoing treatment demand.
-
Rising Geriatric and High-Risk Populations
Older adults and high-risk populations, including immunocompromised individuals, are more susceptible to chronic hepatitis B complications, increasing the need for monitoring and therapy.
-
Technological Innovations in Diagnostics
Development of sensitive and rapid diagnostic tools, including molecular assays and point-of-care tests, enables early detection and monitoring, supporting market expansion.
-
Increased Healthcare Infrastructure in Emerging Markets
Improved access to hepatitis B diagnostics, antiviral treatments, and specialized care facilities in developing countries is enhancing patient reach and driving market growth.
Chronic Hepatitis B Market and Epidemiology Analysis
- Several blood tests are available to diagnose and monitor patients with Chronic Hepatitis B, such as anti-HBc IgG, HBeAg, HBsAg quantitative, ALT, AST, AFP, and others. For chronic cases, a liver biopsy may be needed.
- In 2024, there were approximately 4,970,000 prevalent cases of chronic hepatitis B across the seven major markets (7MM).
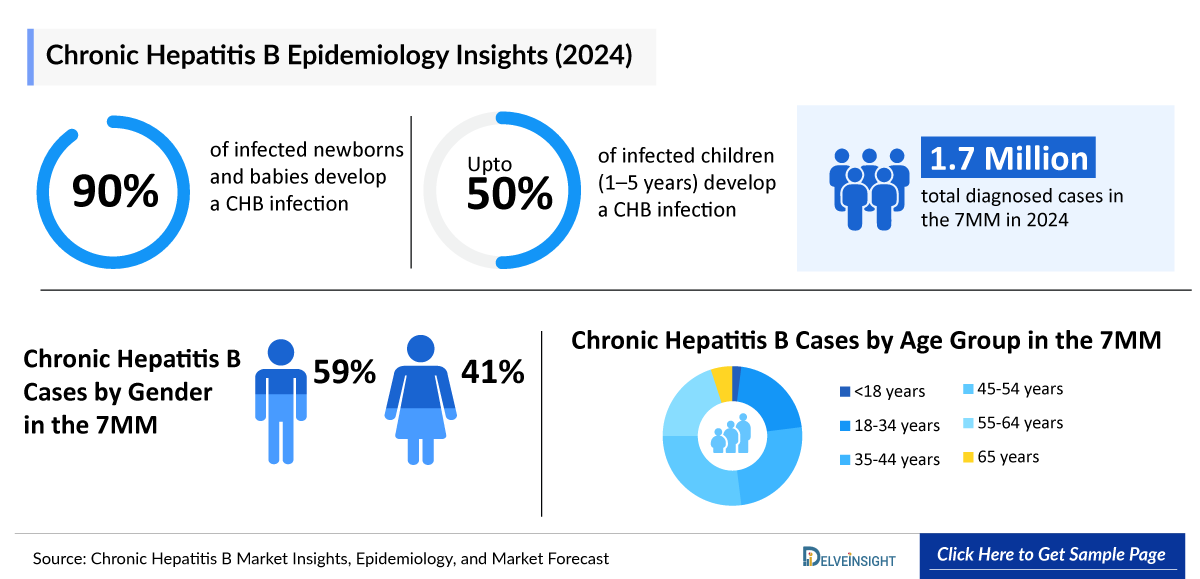
- In 2024, the total diagnosed cases of Chronic Hepatitis B in the 7MM stood at approximately 1,790,000.
- In the 7MM, males accounted for approximately 59% cases and females accounted for almost 41% cases of Chronic Hepatitis B in 2024
- Immunomodulators, such as conventional IFN-a2 and its PEG-IFN-a2, aim to enhance the host immune response but are limited by significant side effects and variable efficacy. NAs, which directly inhibit HBV DNA polymerase, remain the cornerstone of antiviral therapy.
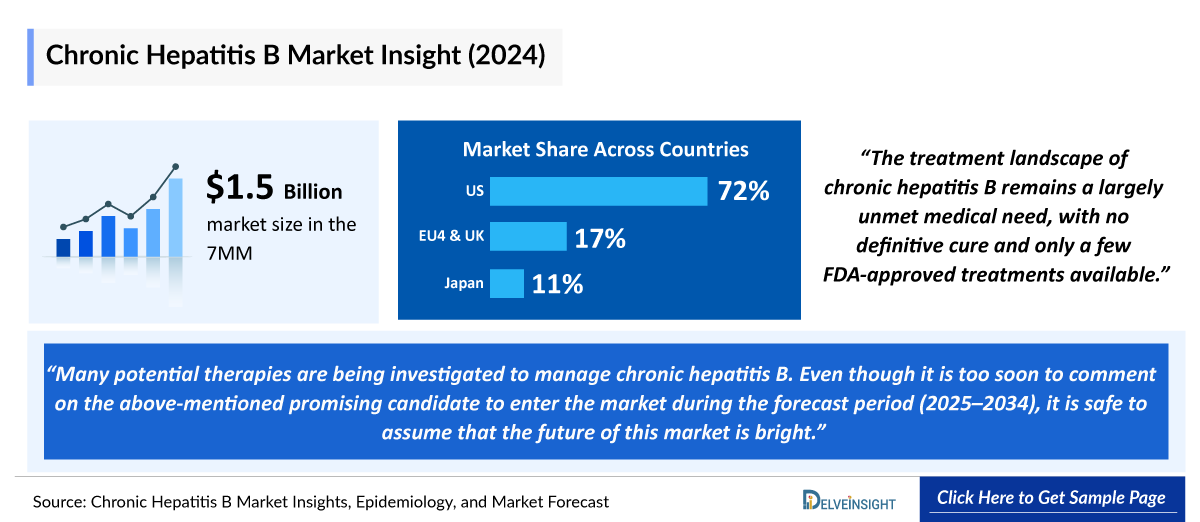
- In 2024, the United States dominated the Chronic Hepatitis B market among the 7MM, capturing approximately 72% of the total market share.
- In 2024, the total market size for Chronic Hepatitis B in EU4 and the UK was approximately 270 USD million, accounting for almost 17% of market in 7MM.
- Meanwhile, the pipeline for Chronic Hepatitis-B therapy continues to expand, with such as Daplusiran/tomligisiran, Imdusiran (AB-729), and others showing promise in clinical development.
- In November 2024, Vir Biotechnology announced positive end-of-treatment results for tobevibart and elebsiran combinations in CHB from the MARCH Study at AASLD, the Liver Meeting.
- In November 2024, GSK presented 12 abstracts at the AASLD, the Liver Meeting 2024, held in San Diego, CA from November 15 to November. The presentations highlighted data from the novel investigational specialty medicine: bepirovirsen, an ASO for Chronic Hepatitis B.
Chronic Hepatitis B Market size and Forecast
- 2025 Chronic Hepatitis B Market Size: USD 1,603 million
- Growth Rate (2025-2034): 12.3% CAGR
- Largest Chronic Hepatitis B Market: United States
Request for unlocking the sample page of the "Chronic Hepatitis B Treatment Market"
The table given below further depicts the key segments provided in the report
Key Factors Driving the Chronic Hepatitis B Market
Growing Chronic Hepatitis B Prevalence
The key strength of CHB as a market opportunity lies in its widespread prevalence and its strong association with serious complications such as liver disease and hepatocellular carcinoma. Approximately 254 million people worldwide are living with chronic hepatitis B, with 1.2 million new infections annually. In 2024, the total diagnosed cases of Chronic Hepatitis B in the 7MM stood at approximately 1.7 million.
Novel Therapeutic Modalities in CHB Management
The development of multiple novel agents, including siRNAs, antisense oligonucleotides, capsid assembly modulators, and immune checkpoint inhibitors, is poised to revolutionize the treatment landscape for CHB.
Launch of Emerging Chronic Hepatitis B Therapies
CHB pipeline possesses some drugs in mid- and late-stage development to be approved in the near future. The expected launch of emerging therapies, such as Imdusiran (AB-729), GSK3228836 (bepirovirsen), Tobevibart (VIR-3434) + elebsiran (VIR-2218) ± PEG-IFN-α), and others, is expected to create a positive impact on the market further.
Chronic Hepatitis B Disease Understanding
Chronic Hepatitis B Overview
Hepatitis B is the most common severe liver infection in the world. It is caused by the hepatitis B virus that attacks and injures the liver. Millions of people are living with chronic hepatitis B infection worldwide. The hepatitis B virus (HBV) is transmitted through blood and infected bodily fluids. It can be passed to others through direct contact with blood, unprotected sex, illegal drugs, and unsterilized or contaminated needles.
Most hepatitis B infections clear up within 1–2 months without treatment. When the infection lasts more than 6 months, it can develop into chronic hepatitis B, leading to chronic inflammation of the liver, cirrhosis (scarring of the liver), liver cancer, and/or liver failure.
The symptoms of hepatitis B include fatigue, poor appetite, stomach pain, fever, nausea, vomiting, and occasionally joint pain, hives, or rash. Urine may become darker, and then jaundice (yellowing of the skin and whites of the eyes) may appear. Adults are more likely than children to develop symptoms.
Chronic hepatitis B infection can last for >6 months (after the first blood test result). This means the immune system cannot get rid of the hepatitis B virus, which remains in the blood and liver. The risk of developing a chronic hepatitis B infection is also directly related to the age at which one first becomes exposed to the hepatitis B virus:
- Nearly 90% of infected newborns and babies develop a chronic hepatitis B infection.
- Up to 50% of infected children (1–5 years) develop a chronic hepatitis B infection.
- About 5–10% of infected adults develop a chronic hepatitis B infection
Further details are provided in the report…
Chronic Hepatitis B Diagnosis
Screening for HBV involves a simple, low-cost blood test to detect hepatitis B surface antigen (HBsAg) and antibodies to HBsAg (anti-HBs) in serum. HBsAg-negative patients who do not have antibodies are given the hepatitis B vaccine. Those who are HBsAg-negative and have detectable anti-HBs are immune (generally from prior vaccination or recovery from an acute infection) and do not require further intervention or testing. A patient who tests positive for HBsAg has an active HBV infection, and further testing is needed to determine the phase of the disease and course of action.
Recommended initial follow-up testing includes serum ALT and HBV DNA levels, serology for hepatitis B e antigen (HBeAg), and antibody to HBeAg (anti-HBe). Individuals with chronic HBV infection have persistently circulating HBsAg in their serum for more than 6 months.
Further details related to country-based variations are provided in the report…
Chronic Hepatitis B Treatment
Hepatitis B infection can result in either an acute or chronic infection. CHB is a blood born virus that primarily affects the liver. When the infection starts to progress after being in the body for 6 months, it is termed “Development or Advancement of Acute Hepatitis B infection into Chronic Hepatitis B infection.” To date, there is no cure for this infection; with the help of antivirals and interferon injections, the virus can be suppressed, or the patient might feel relieved of the symptoms for a specific period; however, there is no permanent solution.
When the hepatitis B virus does not get washed off the body after 6 months, the infection has progressed into the chronic stage, which makes a patient eligible for drug therapy. Usually, drug therapy is used if only one has active liver disease. The US FDA has approved seven drugs to treat hepatitis B. Out of seven, two are given in injectable forms of interferon, while the five other antivirals are tablets. The medications will have to be taken every day without fail; these medicines help suppress the virus’s growth and capability to multiply in the system, followed by a reduction in swelling and liver damage.
Further details related to treatment and management are provided in the report…
Chronic Hepatitis B Epidemiology
The Chronic Hepatitis B (CHB) epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Prevalent Cases of Chronic Hepatitis B, Total Diagnosed Cases of Chronic Hepatitis B, Chronic Hepatitis B cases by Age group, Chronic Hepatitis B cases by Gender, Treated cases of Chronic Hepatitis B, Chronic Hepatitis B cases by impact on Liver in the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In 2024, the total prevalent cases of Chronic Hepatitis B was approximately 2,250,000 in the US.
- In 2024, the total diagnosed cases of Chronic Hepatitis B was approximately 635,000 in the US.
- In 2024, the age group-specific cases of CHB were approximately 10,000, 135,000, 160,000, 170,000, 125,000 and 31,000 cases for <18 years, 18–34 years, 35–44 years, 45–54 years, 55–64 years and =65 years, respectively, in the US.
- In 2024, the compensated liver cases of CHB accounted 85% of all cases and decompensated liver cases of CHB accounted for 15% of the cases in the 7MM.
- In 2024, the treated Cases of Chronic Hepatitis B was approximately 670,000 in the 7MM.
Chronic Hepatitis B Epidemiology Segmentation:
- Total Prevalent Cases of Chronic Hepatitis B
- Total Diagnosed Cases of Chronic Hepatitis B
- Chronic Hepatitis B cases by Age group
- Chronic Hepatitis B cases by Gender
- Treated cases of Chronic Hepatitis B
- Chronic Hepatitis B cases by impact on Liver
Chronic Hepatitis B Recent Developments
- In September 2025, Lion TCR, a clinical-stage biotechnology company, announced that it received Investigational New Drug (IND) clearance from the FDA to begin Phase 1b/2 clinical trials for its proprietary TCR-T cell therapy, LioCyx-M004, in patients with chronic hepatitis B (CHB). This approval marks the third major regulatory milestone for LioCyx-M004, which previously received Fast Track and Orphan Drug Designations for treating hepatitis B virus-related hepatocellular carcinoma (HBV-HCC).
Chronic Hepatitis B Drug Chapters
The section dedicated to drugs in the Chronic Hepatitis B report provides an in-depth evaluation of late-stage pipeline drugs (Phase III and Phase II) related to Chronic Hepatitis B. The drug chapters section provides valuable information on various aspects related to clinical trials of Chronic Hepatitis B, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting Chronic Hepatitis B.
Chronic Hepatitis B Marketed Therapies
- VEMLIDY (tenofovir alafenamide): Gilead Sciences
VEMLIDY is an HBV nucleoside analog reverse transcriptase inhibitor and is indicated for treating chronic hepatitis B virus infection in adults and pediatric patients 12 years and older with compensated liver disease
- In March 2024, Gilead Sciences announced that the US Food and Drug Administration (FDA) approved the supplemental New Drug pplication (sNDA) for VEMLIDY 25 mg tablets as a once-daily treatment for CHB virus infection in pediatric patients 6 years of age and older and weighing at least 25 kg with compensated liver disease.
- In November 2022, the US FDA approved VEMLIDY 25 mg once daily for pediatric patients 12 years and older with chronic HBV infection with compensated liver disease.
- In November 2016, the FDA approved VEMLIDY 25 mg for adults with CHB virus infection with compensated liver disease.
- In January 2017, Gilead Sciences announced that the EC had granted marketing authorization for VEMLIDY (tenofovir alafenamide, TAF) 25 mg to treat CHB infection in adults and adolescents (aged 12 and older with body weight at least 35 kg).
- In December 2016, Gilead Sciences announced that the MHLW had approved VEMLIDY to suppress viral replication in CHB patients with evidence of hepatitis B virus replication and abnormal liver function.
Note: Detailed assessment will be provided in the final report of Chronic Hepatitis B…
Chronic Hepatitis B Emerging Therapies
- Daplusiran/tomligisiran: GSK/Janssen/Arrowhead Pharmaceutical
Daplusiran/tomligisiran (formerly JNJ-3989/GSK5637608) is an investigational HBV-targeted siRNA therapeutic being evaluated in a sequential regimen with bepirovirsen for the treatment of adult non-cirrhotic patients with chronic hepatitis B on NA therapy.
In October 2023, GSK and Arrowhead Pharmaceuticals announced that they had reached an agreement with Janssen Pharmaceuticals to transfer exclusive worldwide rights to further develop and commercialise JNJ-3989 to GSK. JNJ-3989 (formerly ARO-HBV) was initially in-licensed by Janssen from Arrowhead in 2018.
- Imdusiran (AB-729): Arbutus Biopharma
Imdusiran is an RNAi therapeutic specifically designed to reduce all HBV viral proteins and antigens, including HBsAg, which is thought to be a key prerequisite to enable reawakening of a patient’s immune system to respond to the virus. Imdusiran targets hepatocytes using Arbutus’ novel covalently conjugated N-Acetylgalactosamine (GalNAc) delivery technology, enabling subcutaneous delivery.
In the first-half of 2025, the company is planning to initiate a Phase IIb clinical trial of imdusiran combined with IFN and NA therapy.
Chronic Hepatitis B Market Outlook
Treatment landscape of chronic hepatitis B remains a largely unmet medical need, with no definitive cure and only a few FDA-approved treatments available.
- The Chronic Hepatitis B market in the 7MM was valued ~USD 1,603 million in 2025 and is projected to grow with a CAGR of 12.3% over the forecast period from 2025 to 2034.
- Among the 7MM, the United States accounted for the highest market size in 2024, followed by Japan for Chronic Hepatitis B.
- In the EU4 and the UK, Germany has the highest market size of approximately USD 65 million in 2024.
- In 2024, the total market size for Chronic Hepatitis B in Japan was approximately 160 USD million.
- During the forecast period (2024–2034), pipeline candidates such as Daplusiran/tomligisiran, Imdusiran (AB-729), and others are expected to drive the rise in Chronic Hepatitis B market size.
- In a nutshell, many potential therapies are being investigated to manage Chronic Hepatitis B. Even though it is too soon to comment on the above-mentioned promising candidate to enter the market during the forecast period (2025–2034), it is safe to assume that the future of this market is bright. Eventually, these drugs will create a significant difference in the landscape of Chronic Hepatitis B in the coming years. The treatment space is expected to experience a significant positive shift in the coming years owing to the improvement in healthcare spending worldwide.
Further details are provided in the report…
Latest KOL Views on Chronic Hepatitis B
What KOLs are saying on Chronic Hepatitis B Patient Trends?
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of Chronic Hepatitis B (CHB), including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 15 KOLs across the 7MM. We contacted institutions such as the University of Munich, the University of Tokyo, and The Blumberg Institute, University of Minnesota, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Chronic Hepatitis B (CHB) market, which will assist our clients in analyzing the overall epidemiology and market scenario.
Chronic Hepatitis B Report Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in trials for Chronic Hepatitis B (CHB), one of the most important primary endpoints was achieving hemolysis control, LDH normalization, etc. Based on these, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Chronic Hepatitis B Market Access and Reimbursement
Because newly authorized drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of The Chronic Hepatitis B Treatment Market Report
- The Chronic Hepatitis B treatment market report covers a segment of key events, an executive summary, a descriptive overview explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and disease progression, along with country-specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborate profiles of late-stage and prominent therapies, will have an impact on the current Chronic Hepatitis B Treatment Market Landscape.
- A detailed review of the Chronic Hepatitis B Treatment Market, historical and forecasted Chronic Hepatitis B Treatment Market size, Chronic Hepatitis B Drugs Market Share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Chronic Hepatitis B Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Chronic Hepatitis B Drugs Market.
Chronic Hepatitis B Market Report Insights
- Chronic Hepatitis B Patient Population
- Chronic Hepatitis B Therapeutic Approaches
- Chronic Hepatitis B (CHB) Market Size and Trends
- Existing Market Opportunity
Chronic Hepatitis B Market Report Key Strengths
- Ten-year Forecast
- The 7MM Coverage
- Chronic Hepatitis B (CHB) Epidemiology Segmentation
- Key Cross Competition
Chronic Hepatitis B Market Report Assessment
- Current Chronic Hepatitis B Treatment Practices
- Chronic Hepatitis B Market Reimbursements
- Chronic Hepatitis B Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet needs)
- Chronic Hepatitis B Market Drivers
- Chronic Hepatitis B Market Barriers
Key Questions Answered In The Chronic Hepatitis B Market Report:
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Chronic Hepatitis B (CHB) management recommendations?
- Would research and development advances pave the way for future tests and therapies for Chronic Hepatitis B (CHB)?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of Chronic Hepatitis B (CHB)?
- What kind of uptake will the new therapies witness in the coming years in Chronic Hepatitis B (CHB) patients?
Stay Updated with us for Recent Articles



-epidemiology-report.png&w=256&q=75)



