Chronic Plaque Psoriasis Market
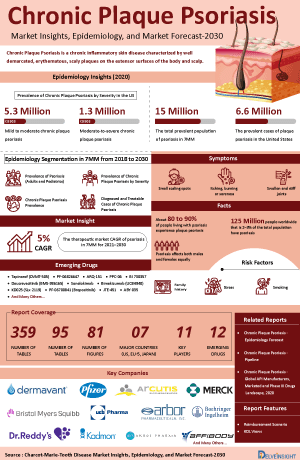
- The Chronic Plaque Psoriasis Market Size in 2023 was approximately USD 14,000 million in the United States. It is expected to grow at a CAGR of 2.8% during the forecast period (2024-2034.)
- In the 7MM, the US accounted for the highest prevalent cases of chronic plaque psoriasis in 2023, with around 6,425,000 diagnosed cases; these numbers are expected to increase during the forecast period (2024-2034).
- Approximately 90% of individuals with psoriasis suffer from psoriasis vulgaris, also known as plaque psoriasis. This form is marked by well-defined, round or oval plaques often covered with silvery white scales.
- Biologic drugs have been a significant advancement in the treatment of plaque psoriasis. These drugs target specific parts of the immune system involved in the development of psoriasis, offering effective control of symptoms for many patients. Drugs such as adalimumab, etanercept, ustekinumab, secukinumab, and ixekizumab have been among the top players in this segment.
- In October 2023, the US FDA approved BIMZELX (bimekizumab) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is the first and only IL-17A and IL-17F inhibitor approved for the treatment of adults with moderate to severe plaque psoriasis.
- Several promising drugs are currently in the pipeline, including ME3183 by Meiji Seika Pharma, SGX302 by Soligenix, and TAK-279 by Takeda.
Request for unlocking the sample page of the "Chronic Plaque Psoriasis Treatment Market"
DelveInsight’s "Chronic Plaque Psoriasis Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of the chronic plaque psoriasis historical and forecasted epidemiology as well as the chronic plaque psoriasis market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Chronic Plaque Psoriasis Treatment Market Report provides current treatment practices, emerging drugs, chronic plaque psoriasis market share of individual therapies, and current and forecasted chronic plaque psoriasis market size from 2020 to 2034, segmented by seven major markets. The report also covers current chronic plaque psoriasis treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Geographies Covered |
|
|
Chronic Plaque Psoriasis Epidemiology |
Segmented by:-
|
|
Chronic Plaque Psoriasis Companies |
|
|
Chronic Plaque Psoriasis Drugs |
|
|
Chronic Plaque Psoriasis Treatment Market |
Segmented by:
|
|
Analysis |
|
Chronic Plaque Psoriasis Treatment Market
Chronic plaque psoriasis is a persistent autoimmune skin condition characterized by raised, red, scaly patches, primarily on the elbows, knees, scalp, and lower back. These plaques result from an accelerated skin cell turnover, leading to a buildup of cells on the skin's surface. The exact cause is unknown, but it involves a combination of genetic and environmental factors, with the immune system mistakenly attacking healthy skin cells. While incurable, the condition can be managed with treatments that reduce inflammation and slow down skin cell production.
Chronic Plaque Psoriasis Diagnosis
Diagnosis of chronic plaque psoriasis is primarily clinical, based on the characteristic appearance of well-demarcated, erythematous plaques topped with silvery scales, commonly located on the elbows, knees, scalp, and lower back. Physicians assess patient history, including any familial predisposition and potential triggers such as stress or infections. While laboratory tests are typically unnecessary for diagnosis, a skin biopsy may be performed in atypical cases to exclude other dermatological conditions. Comprehensive evaluation also considers associated comorbidities like psoriatic arthritis, which may influence management strategies.
Get More Insights of the Report @ Psoriasis Vulgaris Market
Chronic Plaque Psoriasis Treatment
The standard care of treatment for Chronic Plaque Psoriasis includes supportive care, drug therapy, and stem cell transplantation. Chronic Plaque Psoriasis patients with symptoms caused by low blood counts are given supportive care to relieve symptoms and improve their quality of life. While drug therapy may slow the disease’s progression, certain patients can be cured with aggressive treatment with chemotherapy followed by stem cell transplant using stem cells from a donor. Erythropoietin stimulating agents are considered the first-line treatment of low-risk Chronic Plaque Psoriasis patients displaying pretreatment variables predictive of response to treatment.
Further details related to treatment will be provided in the report...
Chronic Plaque Psoriasis Epidemiology
The chronic plaque psoriasis epidemiology chapter in the Chronic Plaque Psoriasis treatment market report provides historical as well as forecasted epidemiology segmented by the Total Prevalent Cases of Plaque Psoriasis, Total Prevalent Cases of Chronic Plaque Psoriasis, Gender-Specific Prevalent cases of Chronic Plaque Psoriasis, Age-Specific Cases of Chronic Plaque Psoriasis, Severity Specific Cases of Chronic Plaque Psoriasis, and Total Treated Cases of Chronic Plaque Psoriasis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In the 7MM, the US accounted for the highest prevalent cases of chronic plaque psoriasis in 2023, with around 6,425,000 cases; these numbers are expected to increase during the forecast period.
- In 2023, nearly 80% cases of chronic plaque psoriasis belonged to mild category.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Chronic Plaque Psoriasis Prevalence
Chronic Plaque Psoriasis Recent Developments
- In May 2025, Samsung Bioepis and Organon announced FDA designated HADLIMA™ (adalimumab-bwwd) autoinjectors and high-concentration prefilled syringe as interchangeable biosimilars to Humira®. Combined with prior approvals, HADLIMA is now interchangeable with all Humira® presentations.
- In May 2025, Arcutis Biotherapeutics (Nasdaq: ARQT) announced FDA approval of the supplemental NDA for ZORYVE® (roflumilast) topical foam 0.3%, now approved to treat scalp and body plaque psoriasis in patients aged 12 and older.
- In May 2025, Fresenius Kabi and Formycon AG announced that the FDA designated Otulfi® (ustekinumab-aauz) as an interchangeable biosimilar to Stelara® (ustekinumab) for treating plaque psoriasis.
- In March 2025, Celltrion announced the U.S. launch of STEQEYMA® (ustekinumab-stba), a biosimilar to STELARA® (ustekinumab), following FDA approval in December 2024. STEQEYMA is approved for the same indications as STELARA, offering consistent treatment for patients and healthcare providers.
- In March 2025, Biocon Biologics Ltd. announced successful results from a pivotal Phase 3, randomized, double-blind, multicenter study comparing Yesintek™ (biosimilar to Ustekinumab) with the reference product Stelara (Ustekinumab) in adults with moderate to severe chronic plaque psoriasis (PsO).
- In December 2024, Celltrion announced that its biosimilar Ustekinumab-stba (Steqeyma) received FDA approval for subcutaneous injection or intravenous infusion. It is approved for the treatment of adult and pediatric patients with plaque psoriasis, arthritis, and psoriatic arthritis, as well as adult patients with Crohn's disease and ulcerative colitis.
Chronic Plaque Psoriasis Drug Chapters
The drug chapter segment of the chronic plaque psoriasis therapeutics market report encloses a detailed analysis of the marketed, late-stage (Phase III), and mid-stage (Phase II) Chronic Plaque Psoriasis pipeline drugs analysis. The marketed drugs segment encloses drugs such as CIMZIA, SILIQ, TALTZ, COSENTYX, OTEZLA and others. Furthermore, the current key players for emerging drugs and their respective drug candidates include ME3183 by Meiji Seika Pharma, SGX302 by Soligenix, and TAK-279 by Takeda and others. The drug chapter also helps understand the chronic plaque psoriasis clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
Chronic Plaque Psoriasis Marketed Drugs
- TALTZ (ixekizumab): Eli Lilly and Company
It is a humanized IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL17A) cytokine and inhibits its interaction with the IL-17 receptor. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. TALTZ inhibits the release of pro-inflammatory cytokines and chemokines. The US FDA approved TALTZ (ixekizumab) injection 80 mg/mL for the treatment of moderate-to-severe plaque psoriasisin adult patients who are candidates for systemic therapy or phototherapy.
- SILIQ (brodalumab): AstraZeneca/Bausch Health
The US FDA approved SILIQ injection for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy and have failed to respond or have lost response to other systemic therapies. Siliq is an IL-17 receptor monoclonal antibody for patients with moderate-to-severe plaque psoriasis, a chronic, debilitating skin disease that causes red patches of skin covered with silvery scales.
|
Comparison of Marketed Drugs | ||||
|
Product |
Company |
MoA |
RoA |
Approval Year (US) |
|
CIMZIA |
UCB Pharma |
Tumor necrosis factor alpha inhibitors |
SC |
2018 (Adults) |
|
SILIQ |
AstraZeneca/Bausch Health |
IL-17A receptor antagonists |
SC |
2017 (Adults) |
|
TALTZ |
Eli Lilly and Company |
IL-17A receptor antagonists |
SC |
2016 (Adults), 2020 (Pediatric) |
|
COSENTYX |
Novartis |
IL-17A protein inhibitors |
SC |
2015 (Adults), 2021 (Pediatrics) |
Chronic Plaque Psoriasis Emerging Drugs
- TAK-279: Takeda
In September 2023, Takeda announced positive topline results from its randomized, double-blind, placebo-controlled, multiple-dose Phase IIb trial evaluating TAK-279, an investigational oral allosteric tyrosine kinase 2 (TYK2) inhibitor with next generation selectivity, in people with active psoriatic arthritis.
Currently, a Phase III trial is being carried out in patients with Plaque Psoriasis to demonstrate how well TAK-279 reduces the skin plaques compared to placebo, in participants with moderate-to-severe plaque psoriasis.
- ME3183: Meiji Seika Pharma
ME3183 is an orally-available and selective PDE4 inhibitor discovered by Meiji. In August 2023, Meiji Seika Pharma announced positive results in the Phase II study of ME3183, a novel highly-potent selective phosphodiesterase-4 (PDE4) inhibitor, in patients with plaque psoriasis conducted in the United States and Canada.
- SGX302: Soligenix
SGX302 is a photodynamic light therapy for psoriasis treatment which uses synthetically manufactured hypericin applied as an ointment and activated with visible fluorescent light. In January 2024, Soligenix announced preliminary top-line results of its ongoing Phase IIa trial of SGX302 (synthetic hypericin) for the treatment of mild-to-moderate psoriasis.
|
Comparison of Emerging Therapies | |||||
|
Emerging Drug |
Company |
Phase |
Molecule Type |
MoA |
RoA |
|
TAK-279 |
Takeda |
III |
Small molecule |
TYK2 inhibitor |
Oral |
|
ME3183 |
Meiji Seika Pharma |
II |
Small molecule |
PDE4 Inhibitor |
Oral |
|
SGX302 |
Soligenix |
II |
Small molecule |
Photodynamic light therapy |
Oral |
Detailed emerging therapies assessment will be provided in the final report...
Chronic Plaque Psoriasis Drugs Market Insights
TNF-alpha inhibitors, including adalimumab (HUMIRA), etanercept (ENBREL), and infliximab (REMICADE), block tumor necrosis factor-alpha, a key cytokine in the inflammatory process of psoriasis. They have been pivotal in treating moderate-to-severe psoriasis and psoriatic arthritis. Despite their effectiveness, these agents carry risks of infections and malignancies, necessitating careful patient selection and monitoring.
IL-17 inhibitors, such as secukinumab (COSENTYX) and ixekizumab (TALTZ), specifically target the IL-17 cytokine, playing a crucial role in psoriasis pathogenesis. These biologics offer rapid and robust efficacy, particularly in difficult-to-treat cases like scalp and nail psoriasis. However, they are associated with risks of candidiasis and potential exacerbation of inflammatory bowel disease.
IL-23 inhibitors, including risankizumab (SKYRIZI) and guselkumab (TREMFYA), selectively block the p19 subunit of IL-23, a cytokine involved in the maintenance of chronic inflammation in psoriasis. These inhibitors provide high levels of skin clearance and are well-tolerated, with the added benefit of less frequent dosing schedules, making them a preferred option for long-term management of moderate-to-severe plaque psoriasis.
Detailed drug class insight assessment will be provided in the final report...
Chronic Plaque Psoriasis Market Outlook
Psoriasis, a chronic relapsing disease, often requires long-term therapy, with treatment choices influenced by disease severity, comorbidities, and healthcare access. Patients are generally categorized as having mild or moderate-to-severe psoriasis. Mild cases are typically treated with topical agents like glucocorticoids, vitamin D analogs, and phototherapy, while moderate-to-severe cases may require systemic treatments, including biologics. Biologics, such as TNF inhibitors (e.g., adalimumab) and interleukin-targeting therapies (e.g., ustekinumab, secukinumab), have emerged as potent options, particularly when traditional therapies fail or are unsuitable. Despite the availability of biosimilars, their uptake, especially for infliximab, has been slow in the US. Studies show that treatment rates for psoriasis hover around 50-60%, with oral therapies gaining traction in recent years. Patients often switch therapies, with adalimumab being a common choice across multiple lines of treatment.
Key Findings
- In the 7MM, the US accounted for the largest Chronic Plaque Psoriasis Market Size in 2023.
- The total Chronic Plaque Psoriasis market size in the US was estimated to be nearly USD 14,000 million in 2023, which is expected to grow during the forecast period (2024–2034).
Chronic Plaque Psoriasis Drugs Uptake
This section focuses on the uptake rate of potential Chronic Plaque Psoriasis drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Chronic Plaque Psoriasis Companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Chronic Plaque Psoriasis Pipeline Development Activities
The report provides insights into Chronic Plaque Psoriasis clinical trials Phase III, Phase II, and Phase I/II. It also analyzes key Chronic Plaque Psoriasis Companies involved in developing targeted therapeutics. Chronic Plaque Psoriasis Companies like Takeda, Meiji Seika Pharma, Soligenix, and others are actively engaging their product in research and development efforts for Chronic Plaque Psoriasis. The Chronic Plaque Psoriasis Pipeline possesses many potential drugs and there is a positive outlook for the therapeutics market, with expectations of growth during the forecast period (2024–2034).
Pipeline Development Activities
The Chronic Plaque Psoriasis treatment market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Chronic Plaque Psoriasis emerging therapy.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Chronic Plaque Psoriasis Treatment Drugs
KOL- Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the Chronic Plaque Psoriasis evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical Directors, Clinical Professors, and Dermatologists, and others.
DelveInsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers such as the Icahn School of Medicine at Mount Sinai, American Academy of Dermatology, International Psoriasis Council, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Chronic Plaque Psoriasis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
KOL Views
“We observed that in 85% of patients, skin psoriasis is the initial manifestation. Frequently, skin psoriasis precedes joint involvement by at least ten years. A typical illustration of this temporal sequence is evident when an individual presents with the skin rash at the age of 18 and subsequently develops joint disease in their mid-30s. However, a smaller proportion of patients experience joint disease as the first symptom, while another group presents with both skin psoriasis and joint involvement concurrently.”
“The new IL-23/Th17 blockers have demonstrated PASI 75 rates of more than 80% in published Phase II studies. So far, new third-generation biologics appear more efficacious and safe because they selectively target the immune system within the skin. Nevertheless, “long-term safety with these drugs remains to be determined. The growing recognition of psoriasis as a systemic condition for dermatologists will probably increase the pressure to treat with more systemic therapies.”
|
KOL Views |
|
“We observed that in 85% of patients, skin psoriasis is the initial manifestation. Frequently, skin psoriasis precedes joint involvement by at least ten years. A typical illustration of this temporal sequence is evident when an individual presents with the skin rash at the age of 18 and subsequently develops joint disease in their mid-30s. However, a smaller proportion of patients experience joint disease as the first symptom, while another group presents with both skin psoriasis and joint involvement concurrently.” |
|
“The new IL-23/Th17 blockers have demonstrated PASI 75 rates of more than 80% in published Phase II studies. So far, new third-generation biologics appear more efficacious and safe because they selectively target the immune system within the skin. Nevertheless, “long-term safety with these drugs remains to be determined. The growing recognition of psoriasis as a systemic condition for dermatologists will probably increase the pressure to treat with more systemic therapies.” |
Chronic Plaque Psoriasis Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Chronic Plaque Psoriasis Therapeutics Market Access and Reimbursement
The treatment and management of Chronic Plaque Psoriasis are expensive. COSENTYX Connect is a personalized support program for people taking or considering COSENTYX. It provides access to a full range of services and support, like its dedicated personal support specialist, a USD 0 copay, and supplemental injection support to make the experience with COSENTYX as easy, affordable, and convenient as possible. The program helps patients with personal support specialists trained to provide supplemental injection support and answer questions about the process started. The personal support specialist or specialty pharmacy will help with the insurance approval process and helps to figure out eligibility for any savings options, including the USD 0 copay program.
Detailed market access and reimbursement assessment will be provided in the final report...
Chronic Plaque Psoriasis Treatment Market Report Scope
- The Chronic Plaque Psoriasis therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Chronic Plaque Psoriasis treatment market landscape.
- A detailed review of the Chronic Plaque Psoriasis treatment market, historical and forecasted Chronic Plaque Psoriasis market size, Chronic Plaque Psoriasis market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Chronic Plaque Psoriasis treatment market report provides an edge while developing business strategies by understanding trends through SWOT analysis and KOL views, patient journey, and treatment preferences that help shape and drive Chronic Plaque Psoriasis.
Chronic Plaque Psoriasis Treatment Market Report Insights
- Patient-based Chronic Plaque Psoriasis Market Forecasting
- Chronic Plaque Psoriasis Therapeutic Approaches
- Chronic Plaque Psoriasis Pipeline Analysis
- Chronic Plaque Psoriasis Treatment Market Size and Trends
- Existing and Future Chronic Plaque Psoriasis Drugs Market Opportunity
Chronic Plaque Psoriasis Treatment Market Report Key Strengths
- 11 Years Chronic Plaque Psoriasis Market Forecast
- The 7MM Coverage
- Chronic Plaque Psoriasis Epidemiology Segmentation
- Key Cross Competition
- Chronic Plaque Psoriasis Drugs Uptake
- Key Chronic Plaque Psoriasis Market Forecast Assumptions
Chronic Plaque Psoriasis Treatment Market Report Assessment
- Current Chronic Plaque Psoriasis Treatment Market Practices
- Chronic Plaque Psoriasis Unmet Needs
- Chronic Plaque Psoriasis Pipeline Product Profiles
- Chronic Plaque Psoriasis Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Chronic Plaque Psoriasis Market Drivers
- Chronic Plaque Psoriasis Market Barriers
FAQs
- What was the Chronic Plaque Psoriasis treatment market size, the Chronic Plaque Psoriasis treatment market size by therapies, Chronic Plaque Psoriasis drugs market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for Chronic Plaque Psoriasis?
- What are the disease risks, burdens, and Chronic Plaque Psoriasis unmet needs? What will be the growth opportunities across the 7MM concerning the patient population with Chronic Plaque Psoriasis?
- What are the current options for the Chronic Plaque Psoriasis treatment? What are the current guidelines for treating Chronic Plaque Psoriasis in the 7MM?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What is the patient share in Chronic Plaque Psoriasis?
Reasons to Buy Chronic Plaque Psoriasis Drugs Market Report
- The Chronic Plaque Psoriasis treatment market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Chronic Plaque Psoriasis drugs market.
- Insights on patient burden/disease Chronic Plaque Psoriasis prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Chronic Plaque Psoriasis treatment market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the Chronic Plaque Psoriasis treatment market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of current therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles