Chronic Rhinosinusitis With Nasal Polyps Market
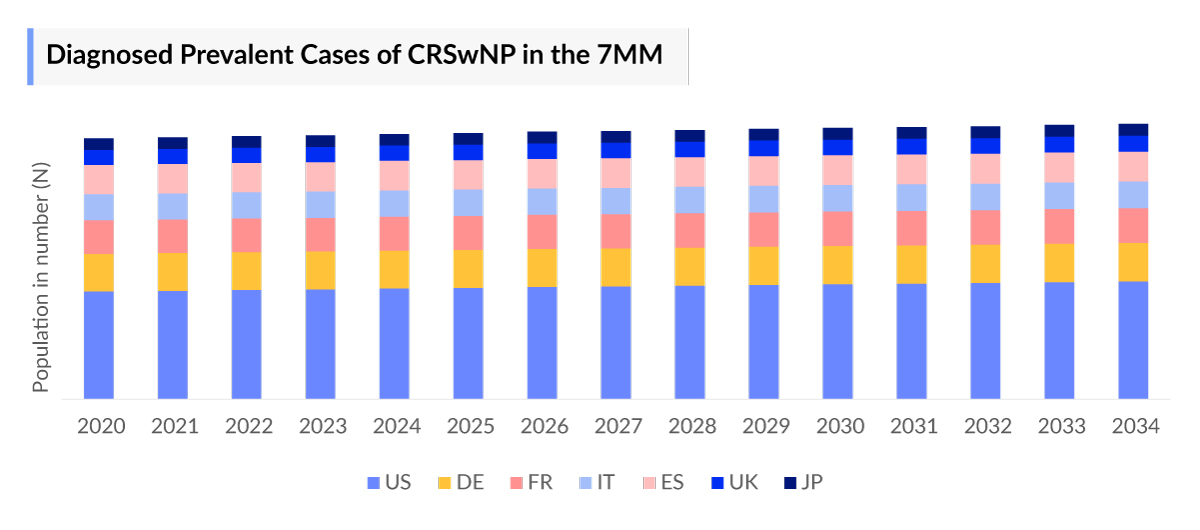
- According to DelveInsight’s estimates, the total CRSwNP diagnosed prevalent cases in the 7MM, were ~4,404 thousand in 2023.
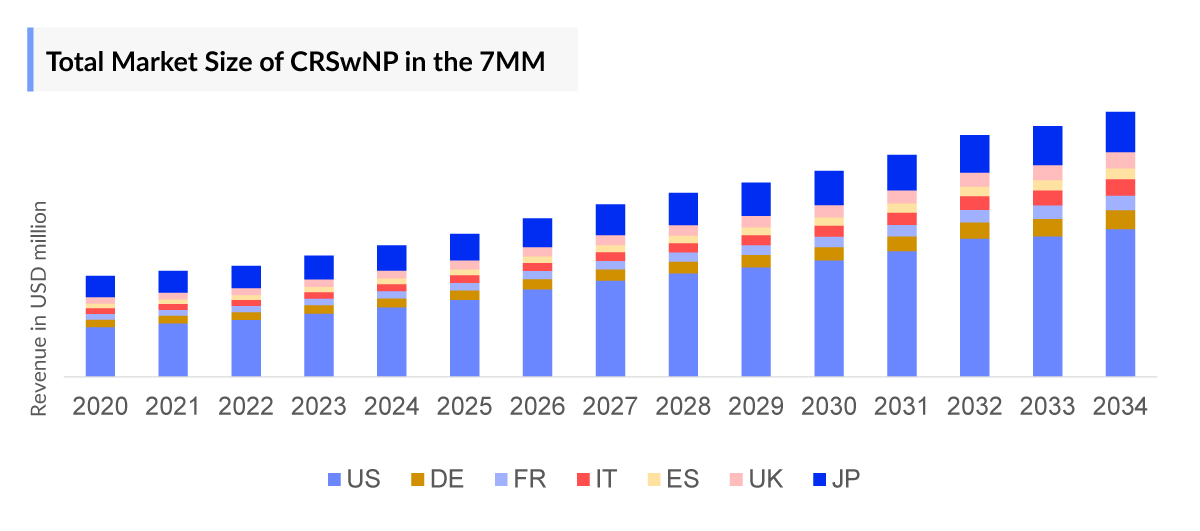
- In 2023, the market size of CRSwNP was highest in the US among the 7MM, accounting for approximately USD 1,674 million which is further expected to increase by 2034 at a CAGR of 10.8%.
- The current Chronic Rhinosinusitis with Nasal Polyps Treatment includes Corticosteroids (first line and second line), DUPIXENT, NUCALA, XOLAIR, and others, an approved drug for the treatment of Chronic Rhinosinusitis with Nasal Polyps.
- The different pharmacological therapies used to treat CRSwNP belong mainly to classes of first-line treatment and refractory treatment, used for the management of the disease.
- In May 2024, the US Food and Drug Administration (FDA) accepted for Priority Review the supplemental Biologics License Application (sBLA) for DUPIXENT (dupilumab) (product of Sanofi and Regeneron Pharmaceuticals) as an add-on maintenance treatment for adolescents aged 12 to 17 years with inadequately controlled chronic rhinosinusitis with nasal polyposis (CRSwNP). The target action date for the FDA decision is September 15, 2024.
- The emerging drug FASENRA is expected to launch in the US by 2025, in EU4 and the UK by 2026, and in Japan by 2027, which has the potential to reduce the disease burden of Chronic Rhinosinusitis with Nasal Polyps in the forecasted years.
- In May 2024, AstraZeneca published latest research across key respiratory and immune-mediated diseases at ATS 2024 showcasing strength of its broad pipeline and portfolio. AstraZeneca demonstrated that FASENRA is in development for chronic rhinosinusitis with nasal polyps and other diseases.
Request for Sample Page of the "Chronic Rhinosinusitis with Nasal Polyps Drugs Market"
DelveInsight’s “Chronic Rhinosinusitis with Nasal Polyps Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of CRSwNP, historical and forecasted epidemiology as well as the Chronic Rhinosinusitis with Nasal Polyps market trends in the United States, EU4, and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The CRSwNP Drugs Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Chronic Rhinosinusitis with Nasal Polyps market size from 2020 to 2034. The Report also covers current Chronic Rhinosinusitis with Nasal Polyps Treatment Market practices, Chronic Rhinosinusitis with Nasal Polyps Market Drivers, market barriers, SWOT analysis, reimbursement and market access, and Chronic Rhinosinusitis with Nasal Polyps unmet needs to curate the best of the opportunities and assess the underlying potential of the market.
Chronic Rhinosinusitis with Nasal Polyps Treatment Market
Nasal Polyposis/Nasal polyps is small, benign drop-like growths appearing in the mucosa (lining tissues) of the nose and may block the nasal passageway. Nasal polyps are a subgroup of chronic rhinosinusitis. Chronic rhinosinusitis is a condition where the nasal cavity and sinuses are inflamed for more than 4 to 12 weeks. Patients suffering from chronic rhinosinusitis condition can be progressed into Chronic Rhinosinusitis with Nasal Polyps, and chronic rhinosinusitis without NP (CRSsNP). Only a portion of chronic rhinosinusitis will develop nasal polyps.
CRSwNP is a potentially debilitating condition in adults that are characterized by inflammation of the nose and paranasal sinuses with the presence of nasal polyps. People with a history of chronic sinus infections, allergic rhinitis, asthma, and cystic fibrosis, sensitivity to NSAIDs like ibuprofen and aspirin, and Churg–Strauss syndrome are more prone to get nasal polyps. Patients suffering from nasal polyps may experience chronic nasal congestion, running nose, postnasal drip, reduced sense of smell and taste, difficulty in breathing, pressure in the face or forehead area, snoring, sleep apnea, and others.
Chronic Rhinosinusitis with Nasal Polyps Diagnosis
For the diagnosis of nasal polyps, an ENT specialist or any other physician first looks into the nasal passages with a special lighted instrument known as a nasoscope. The most preferred techniques are confirming the location, size, and severity of polyps nasal endoscopy, and radiological imaging. Further allergy tests and blood tests can be done to determine other disease-related components.
Further details related to diagnosis are provided in the report…
Chronic Rhinosinusitis with Nasal Polyps Treatment
Corticosteroids are considered the first-line therapy for the treatment of nasal polyps. Initially, nasal corticosteroids are recommended for treatment. If the patient does not progress, they may be shifted to oral corticosteroids and then to injectable corticosteroids. If the corticosteroids do not show progress in the condition, surgical intervention is the next treatment of choice. Biologics can be given to treat severe polyps, treat post-surgery reoccurrence cases, and treat patients who cannot undergo surgery. Anti-allergic NSAIDs can be added to the regimen depending on the condition.
The currently recommended range of pharmacological treatments includes mainly biologics and corticosteroids, including NUCALA (mepolizumab), DUPIXENT (dupilumab), XOLAIR (omalizumab), XHANCE (fluticasone propionate), SINUVA (mometasone furoate) Sinus Implant, and PROPEL (mometasone furoate) as currently approved for treating nasal polyps and CRSwNP. With many treatment options already present, the market anticipates the launch of emerging products with novel CRSwNP MoA targets and the onset of action.
CRSwNP is a complex and frequent respiratory condition that poses significant challenges to both the patients who experience it and the physicians who treat them. Treatment aims to eliminate or significantly reduce the size of the nasal polyps, resulting in relief of nasal obstruction, improvement in sinus drainage, and restoration of olfaction and taste. A handful of clinical trials are expected to change the landscape of the emerging therapeutic market in the forecast period. Profound disease understanding, development of novel therapeutic agents, and patient-centered treatment approaches will improve patient outcomes in the coming years.
Further details related to treatment are provided in the report…
Chronic Rhinosinusitis with Nasal Polyps Epidemiology
As the market is derived using a patient-based model, the CRSwNP epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Prevalent cases of Chronic Rhinosinusitis (CRS), Diagnosed prevalent cases of CRS, Diagnosed prevalent cases of Chronic Rhinosinusitis with Nasal Polyps, and Gender-specific diagnosed prevalent cases of Chronic Rhinosinusitis with Nasal Polyps, in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- In the assessment done by DelveInsight, the estimated total Chronic Rhinosinusitis Prevalent Cases in the 7MM were ~70,188 thousand in 2023.
- The highest total Chronic Rhinosinusitis with Nasal Polyps diagnosed prevalent cases were accounted by the US in 2023 (~2,209 thousand), which is expected to show a rise in the future.
- Among the European countries, Germany had the highest Chronic Rhinosinusitis with Nasal Polyps diagnosed prevalent cases with ~616 thousand cases, followed by the UK, which had diagnosed prevalent population of ~441 thousand in 2023. On the other hand, Spain had the lowest diagnosed prevalent population (~189 thousand cases).
- Japan had ~202 thousand total diagnosed prevalent cases of Chronic Rhinosinusitis with Nasal Polyps in 2023, accounting for approximately 5% in 7MM.
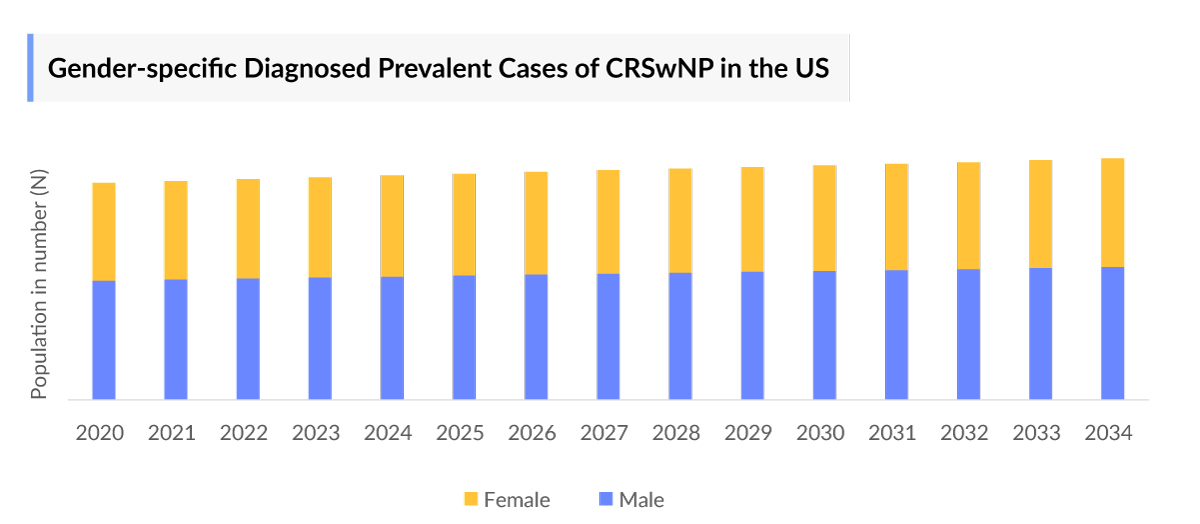
- In 2023, in the US, the gender-specific diagnosed prevalent cases of Chronic Rhinosinusitis with Nasal Polyps were highest for males (~55%), as compared to males (~45%), which is attributed to factors such as hormonal differences and physiology, lifestyle and occupational factors and others.
Chronic Rhinosinusitis with Nasal Polyps Recent Developments
- In January 2025, GSK's IL-5 inhibitor Nucala was approved in China for the treatment of individuals with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP), a condition affecting nearly 30 million people in the country.
- On November 11, 2024, AstraZeneca and Amgen announced that their subcutaneous antibody Tezspire (tezepelumab) met the primary endpoint in the Phase III WAYPOINT study, showing significant efficacy in patients with chronic rhinosinusitis with nasal polyps.
Chronic Rhinosinusitis with Nasal Polyps Drug Chapters
The drug chapter segment of the CRSwNP Drugs Market Report encloses a detailed analysis of Chronic Rhinosinusitis with Nasal Polyps off-label drugs and late-stage (Phase-III and Phase-II) Chronic Rhinosinusitis with Nasal Polyps pipeline drugs. It also helps to understand the Chronic Rhinosinusitis with Nasal Polyps clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Chronic Rhinosinusitis with Nasal Polyps Marketed Drugs
- NUCALA (mepolizumab): GlaxoSmithKline
GSK’s NUCALA (mepolizumab) is the first IL-5 therapy approved as an add-on treatment in the US for adults with CRSwNP to target eosinophilic inflammation in adult patients 18 years of age and older with inadequate response to nasal corticosteroids. The recommended dosage of mepolizumab is 100 mg, administered once every 4 weeks by subcutaneous injection into the upper arm, thigh, or abdomen.
Mepolizumab is an IL-5 antagonist (IgG1 kappa). IL-5 is the major cytokine responsible for the growth and differentiation, recruitment, activation, and survival of eosinophils. Mepolizumab binds to IL-5 with a dissociation constant of 100 pM, inhibiting the bioactivity of IL-5 by blocking its binding to the alpha chain of the IL-5 receptor complex expressed on the eosinophil cell surface. By inhibiting IL-5 signaling, mepolizumab reduces the production and survival of eosinophils; however, the mechanism of mepolizumab action in asthma, CRSwNP, EGPA, and HES has not been definitively established.
The US FDA approved NUCALA in July 2021 based on positive results from the pivotal SYNAPSE study.
For More Information @ Mepolizumab Market
Chronic Rhinosinusitis with Nasal Polyps Emerging Drugs
- TEZSPIRE (tezepelumab): AstraZeneca/Amgen
TEZSPIRE (tezepelumab) is a potential human monoclonal antibody (immunoglobulin [Ig] G2λ) that inhibits the action of thymic stromal lymphopoietin (TSLP). TSLP expression is elevated in the airways of patients with asthma compared with healthy individuals and in nasal polyp tissue from patients with CRSwNP compared with healthy sinus tissue or patients with CRS without NP. Tezepelumab binds specifically to TSLP, preventing its interaction with its heterodimeric receptor.
Tezepelumab is currently under Phase III clinical trials to evaluate the efficacy and safety of tezepelumab in adults with severe CRSwNP. This trial is based on positive results of tezepelumab from the PATHWAY and NAVIGATOR trials in patients with severe, uncontrolled asthma and comorbid nasal polyps.
Get More Insights @ TEZEPELUMA Market
- Depemokimab/GSK3511294: GlaxoSmithKline
Depemokimab/GSK3511294, which GSK is developing to treat CRSwNP, is a humanized anti-interleukin (IL)-5 monoclonal antibody. As a new biological entity, it is engineered to ensure high affinity and long-acting suppression of IL-5 functions. IL-5 is cytokines responsible for the proliferation, activation, and survival of eosinophils, thus making them a proven treatment target for diseases with higher levels of eosinophils.
Depemokimab, with an extended half-life and improved IL-5 affinity compared to other approved therapies, is being studied by GSK in Phase III to evaluate the efficacy and safety of depemokimab in participants with CRSwNP.
- FASENRA (benralizumab): AstraZeneca
FASENRA (benralizumab), being developed by AstraZeneca, is a monoclonal antibody that binds directly to interleukin-5 receptor alpha (IL-5Rα) on eosinophils and attracts natural killer cells to induce rapid and near-complete depletion of eosinophils via apoptosis. FASENRA targets seven diseases beyond respiratory disease.
IL-5 induces an eosinophil-mediated inflammatory response by binding to the IL-5R expressed in eosinophils, basophils, and some mast cells. Benralizumab, unlike IL-5 low-affinity binding, binds with high affinity to the domain I of the α-chain of IL-5R and blocks its signaling and the proliferation of IL-5-dependent cell lines. On the other hand, benralizumab is an afucosylated antibody in the CH2 region, giving it a high affinity for the FcγRIIIa on natural killer cells, macrophages, and neutrophils; this binding triggers a magnified apoptosis response in eosinophils via antibody-dependent cell-mediated cytotoxicity. It is currently being evaluated in Phase III clinical trials for treating nasal polyposis.
FASENRA, has an immediate competitor in the same class, i.e., NUCALA, the first IL-5 inhibitor approved for nasal polyps developed by GSK. Except for NUCALA, Sanofi and Regeneron’s DUPIXENT is another biologic present in the market to compete with FASENRA.
Watch More Insights @ FASENRA (Benralizumab) Market
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
TEZSPIRE |
Anti-TSLP |
SC |
AstraZeneca |
III | |
|
XXX |
XXX |
XXX |
XXX |
II |
Note: Detailed emerging therapies assessment will be provided in the final report of Chronic Rhinosinusitis with Nasal Polyps.
Chronic Rhinosinusitis with Nasal Polyps Market Outlook
The Chronic Rhinosinusitis with Nasal Polyps Market Outlook is anticipated to evolve largely due to the quick approvals of new therapies by regulatory agencies like the European Commission and the US FDA. The current market for CRSwNP is undergoing a boom with the approval of biologics that target uncontrolled severe nasal polyps cases and can be given post-surgery in the recurrence of Nasal polyp cases.
Various FDA-approved therapies by major key pharmaceutical players are available in the Chronic Rhinosinusitis with Nasal Polyps Drugs Market, including branded biologics that include NUCALA (mepolizumab), DUPIXENT (dupilumab), and XOLAIR (omalizumab), along with other treatment options like XHANCE (fluticasone propionate), SINUVA (mometasone furoate) Sinus Implant, and PROPEL (mometasone furoate). Among the 7MM, the major share of the market was captured by Corticosteroids.
With a handful number of clinical trials in the CRSwNP emerging pipeline, TEZSPIRE is under development by AstraZeneca and Amgen for treating CRSwNP and is currently in Phase III of development for CRSwNP treatment along with other emerging options. Considering the treatment paradigm for CRSwNP, a substantial market has been designated toward biologics where many potential candidates like DUPIXENT and others have marked their coveted entries; the market looks forward to catering to other biologics under development.
In the upcoming Chronic Rhinosinusitis with Nasal Polyps treatment market landscape, there are a plethora of companies investigating agents for use in Chronic Rhinosinusitis with Nasal Polyps which includes AstraZeneca, Amgen and others. There are many more pharma companies which are conducting clinical trials for therapies for Chronic Rhinosinusitis with Nasal Polyps.
- The Chronic Rhinosinusitis with Nasal Polyps Therapeutics Market Size in the 7MM was approximately USD 2,249 million in 2023, the market is expected to grow at a Compound annual growth rate (CAGR) of 7.18% during the forecast period (2024–2034) attributed to the biologic therapies, and others.
- The United States accounted for the highest Chronic Rhinosinusitis with Nasal Polyps Market Size approximately 74% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the European countries, Germany had the highest Chronic Rhinosinusitis with Nasal Polyps market size with nearly USD 161 million in 2023, while Spain had the lowest market size of Chronic Rhinosinusitis with Nasal Polyps with nearly USD 44 million in 2023.
- The Chronic Rhinosinusitis with Nasal Polyps Market Size in Japan was estimated to be nearly USD 88 million in 2023, which accounts for 4% of the total 7MM market.
- With the expected launch of upcoming therapies, such as TEZSPIRE (tezepelumab) the total market size of Chronic Rhinosinusitis with Nasal Polyps is expected to show change in the upcoming years.
Chronic Rhinosinusitis with Nasal Polyps Drugs Uptake
This section focuses on the uptake rate of potential Chronic Rhinosinusitis with Nasal Polyps drugs expected to launch in the market during 2020–2034. For example, TEZSPIRE (tezepelumab) in the US is expected to be launched by 2025 with a peak share of 3.2%. TEZSPIRE is anticipated to take 7 years to peak with a medium uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Chronic Rhinosinusitis with Nasal Polyps Pipeline Development Activities
The Chronic Rhinosinusitis with Nasal Polyps pipeline report provides insights into different Chronic Rhinosinusitis with Nasal Polyps clinical trials within Phase III, Phase II, and Phase I. It also analyzes key Chronic Rhinosinusitis with Nasal Polyps Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Chronic Rhinosinusitis with Nasal Polyps clinical trials report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Chronic Rhinosinusitis with Nasal Polyps emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Chronic Rhinosinusitis with Nasal Polyps evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from Wright State University Boonshoft School of Medicine, Fairborn, US; University of California San Diego, San Diego, California, US; Department of Otorhinolaryngology–Head and Neck Surgery, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, US; University of Siena, Italy; University of Southampton, Southampton, UK; University of Fukui, Fukui, Japan; Nippon Medical School, Tokyo, Japan; and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Chronic Rhinosinusitis with Nasal Polyps Therapeutis Market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and Chronic Rhinosinusitis with Nasal Polyps Drugs Market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Chronic Rhinosinusitis with Nasal Polyps Drugs Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The Chronic Rhinosinusitis with Nasal Polyps Drugs Market Report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Chronic Rhinosinusitis with Nasal Polyps Therapeutics Market Report Scope
- The CRSwNP Therapeutics Market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the Chronic Rhinosinusitis with Nasal Polyps epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Chronic Rhinosinusitis with Nasal Polyps Therapeutics Market, historical and forecasted Chronic Rhinosinusitis with Nasal Polyps market size, Chronic Rhinosinusitis with Nasal Polyps market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The Chronic Rhinosinusitis with Nasal Polyps Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Chronic Rhinosinusitis with Nasal Polyps Drugs Market.
Chronic Rhinosinusitis with Nasal Polyps Therapeutics Market Report Insights
- Patient-based CRSwNP Market Forecsting
- CRSwNP Therapeutic Approaches
- CRSwNP Pipeline Analysis
- CRSwNP Market Size
- CRSwNP Market Trends
- Existing and Future CRSwNP Therapeutics Market Opportunities
Chronic Rhinosinusitis with Nasal Polyps Therapeutics Market Report Key Strengths
- 11 years Chronic Rhinosinusitis with Nasal Polyps Market Forecast
- The 7MM Coverage
- CRSwNP Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- CRSwNP Drugs Uptake
- Key CRSwNP Market Forecast Assumptions
Chronic Rhinosinusitis with Nasal Polyps Therapeutics Market Report Assessment
- Current CRSwNP Treatment Market Practices
- CRSwNP Unmet Needs
- CRSwNP Pipeline Product Profiles
- CRSwNP Treatment Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- CRSwNP Market Barriers
- CRSwNP Market Barriers
Key Questions Answered In The CRSwNP Market Report:
CRSwNP Treatment Market Insights
- What was the CRSwNP market share (%) distribution in 2020 and what it would look like in 2034?
- What would be the Chronic Rhinosinusitis with Nasal Polyps market size as well as market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Chronic Rhinosinusitis with Nasal Polyps market size during the forecast period (2024–2034)?
- At what CAGR, the Chronic Rhinosinusitis with Nasal Polyps Treatment Market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the Chronic Rhinosinusitis with Nasal Polyps Market Outlook across the 7MM during the forecast period (2024–2034)?
- What would be the Chronic Rhinosinusitis with Nasal Polyps Market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
CRSwNP Epidemiology Insights
- What are the disease risk, burden, and Chronic Rhinosinusitis with Nasal Polyps Unmet Needs?
- What is the historical Chronic Rhinosinusitis with Nasal Polyps patient population in the United States, EU4 (Germany, France, Italy, Spain) and the UK, and Japan?
- What would be the forecasted patient population of Chronic Rhinosinusitis with Nasal Polyps at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Chronic Rhinosinusitis with Nasal Polyps?
- Out of the above-mentioned countries, which country would have the highest prevalent population of Chronic Rhinosinusitis with Nasal Polyps during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current CRSwNP Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of CRSwNP along with the approved therapy?
- What are the current treatment guidelines for the treatment of Chronic Rhinosinusitis with Nasal Polyps in the US, Europe, And Japan?
- What are the CRSwNP marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many CRSwNP companies are developing therapies for the treatment of Chronic Rhinosinusitis with Nasal Polyps?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Chronic Rhinosinusitis with Nasal Polyps?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to the CRSwNP therapies?
- What are the recent therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the clinical studies going on for CRSwNP and their status?
- What are the key designations that have been granted for the Chronic Rhinosinusitis with Nasal Polyps Emerging Therapies?
- What are the 7MM historical and forecasted CRSwNP Treatment Market?
Reasons to Buy CRSwNP Market Forecast Report
- The Chronic Rhinosinusitis with Nasal Polyps Drugs Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Chronic Rhinosinusitis with Nasal Polyps Treatment Market.
- Insights on patient burden/disease Chronic Rhinosinusitis with Nasal Polyps Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Chronic Rhinosinusitis with Nasal Polyps Drugs Market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Chronic Rhinosinusitis with Nasal Polyps Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for New Articles:- Latest DelveInsight Blogs