Chronic Thromboembolic Pulmonary Hypertension Market
Key Highlights
- The Chronic Thromboembolic Pulmonary Hypertension (CTEPH) market size is expected to have steady growth during the forecast period (2024–2034). This growth is a direct consequence of increasing disease awareness and CTEPH prevalence rate, improved diagnostic practices, greater availability of therapies, and the emergence of new therapies.
- Though the mainstay treatment for CTEPH is pulmonary endarterectomy, for inoperable or residual cases, ADEMPAS (riociguat) by Bayer is approved. It dominates the market due to its dual advantage of being the only approved product having a first-mover advantage. The current landscape also has some off-label drugs, like endothelin receptor antagonists, prostacyclin analogs, and phosphodiesterase type 5 inhibitors, anticoagulants.
- The CTEPH pipeline is limited with only a few companies like Sci Pharma developing drugs. The CTEPH market size is anticipated to grow at a significant CAGR during the forecast period (2024–2034) propelled by increasing disease prevalence, growth in market size of current therapy, and projected entry of emerging therapy
DelveInsight’s comprehensive report titled “Chronic Thromboembolic Pulmonary Hypertension (CTEPH) — Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of CTEPH. The report presents historical and projected epidemiological data covering total diagnosed prevalent cases of CTEPH, gender-specific cases of CTEPH, age-specific cases of CTEPH, and treated cases of CTEPH. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing treatment practices and unmet medical requirements in chronic thromboembolic pulmonary hypertension. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
Chronic Thromboembolic Pulmonary Hypertension Overview
CTEPH is a rare, progressive pulmonary vascular disease characterized by macroscopic thromboembolic lesions (blood clots) and microscopic pulmonary vascular changes. Clinically, it is defined as a mean pulmonary artery pressure ≥25 mmHg at rest persisting for at least 6 weeks in patients with previous pulmonary artery embolism.
CTEPH results from incomplete resolution of acute pulmonary emboli, leading to organized fibrotic material within the pulmonary vasculature. This fibrotic material obstructs blood flow, increases pulmonary vascular resistance, and ultimately leads to right heart failure. The symptoms of CTEPH include shortness of breath, edema, fatigue, and chest pain and are therefore similar to other, more common diseases, resulting in an often delayed diagnosis of CTEPH. Symptoms can vary depending on the extent and severity of pulmonary vascular obstruction.
Chronic Thromboembolic Pulmonary Hypertension Diagnosis and Treatment Algorithm
The diagnosis of CTEPH requires a high index of suspicion. Imaging modalities such as ventilation/perfusion (V/Q) lung scans and pulmonary angiography are essential for diagnosis. Echocardiography and right heart catheterization are used to assess the severity of PH and right heart function.
The mainstay of treatment for CTEPH is pulmonary endarterectomy (PEA), a surgical procedure aimed at removing organized thromboembolic material from the pulmonary arteries. Inoperable or residual disease after PEA may be managed with medical therapy, including pulmonary vasodilators such as riociguat, which has been shown to improve exercise capacity and hemodynamics in CTEPH patients.
Treatment aims to improve pulmonary hemodynamics and quality of life, with adjunctive measures such as diuretics and oxygen therapy. Currently, there is only one approved drug for the optimal medical treatment of CTEPH which consists of ADEMPAS (riociguat) from Bayer. Certain off-label drugs are also given to patients having CTEPH such as anticoagulants, diuretics, endothelin receptor antagonists, prostacyclin analogs, and phosphodiesterase type 5 inhibitors in case of heart failure or hypoxemia. Lifelong anticoagulation is recommended, even after PEA, though no data exist on the efficacy and safety of new oral anticoagulants. Pharmaceutical treatments help ameliorate behavioral symptoms of CTEPH, including irritability, aggression, and self-injurious behavior.
Chronic Thromboembolic Pulmonary Hypertension Epidemiology
The epidemiology section on the chronic thromboembolic pulmonary hypertension market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of chronic thromboembolic pulmonary hypertension (CTEPH). Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
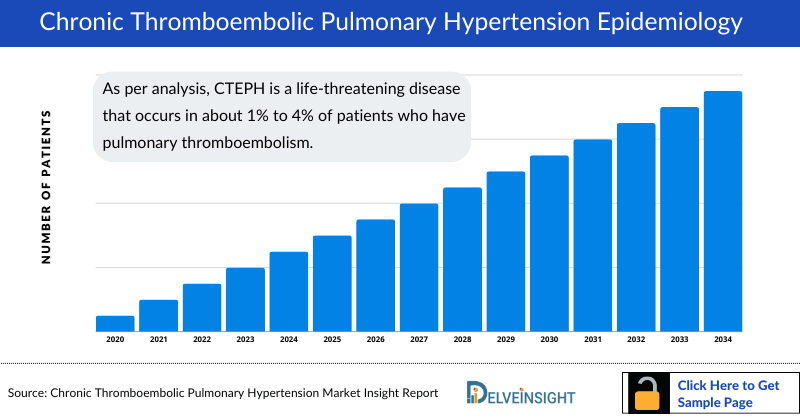
- As per analysis, CTEPH is a life-threatening disease that occurs in about 1% to 4% of patients who have pulmonary thromboembolism. A certain proportion of patients diagnosed with acute pulmonary embolism progress to and develop CTEPH.
- The rate of CTEPH diagnosis varies from approximately 10% in Europe to about 30% in the US. Based on our analysis it is estimated that prevalence estimates range from 3 to 30 cases per million individuals. It is challenging to assess the actual prevalence, due to its rarity and potential underdiagnosis. But despite its rarity, CTEPH is associated with a high mortality rate.
- As per analysis, women accounted for a higher percentage of CTEPH prevalent cases approximately 50% percent, compared to males with approximately 45%. The analysis also suggests age dependence, with a peak in CTEPH cases among older individuals around 70 years, especially those associated with other medical conditions.
Chronic Thromboembolic Pulmonary Hypertension Market Outlook
Chronic Thromboembolic Pulmonary Hypertension market share is estimated to grow during the forecasted period. The major factors driving the positive chronic thromboembolic pulmonary hypertension (CTEPH) market outlook are increasing disease awareness and prevalence rate, improved diagnostic practices, and approvals for the treatment of CTEPH, apart from conventional PTE surgery. The primary treatment for CTEPH is PTE surgery which can potentially cure the condition and is considered the treatment of choice.
However, not all chronic thromboembolic pulmonary hypertension patients are suitable for PTE surgery, due to the location or extent of the thromboembolic material, or the presence of comorbidities. In those cases, medical therapies are the main treatment approach.
The medical treatment of chronic thromboembolic pulmonary hypertension includes the use of anticoagulants, and therapies recommended for pulmonary arterial hypertension such as endothelin receptor antagonists, phosphodiesterase type-5 inhibitors, and the soluble guanylate cyclase stimulators like ADEMPAS (riociguat), the only approved medication for chronic thromboembolic pulmonary hypertension.
In addition, for chronic thromboembolic pulmonary hypertension patients who are not candidates for PTE, other interventional procedures like balloon pulmonary angioplasty and lung transplants may be considered. Balloon pulmonary angioplasty has recently emerged as an alternative intervention for non-surgical candidates with CTEPH. If the patient is neither a candidate for PTE surgery nor balloon angioplasty, lung transplantation may be an option.
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the CTEPH market in the 7MM is expected to change significantly during the study period 2020–2034.
Chronic Thromboembolic Pulmonary Hypertension Recent Developments
- In March 2025, SCIENTURE HOLDINGS, INC. announced it completed a draw on its Equity Line of Credit (ELOC) to support the commercial launch of Arbli™ (losartan potassium) Oral Suspension. The company also temporarily suspended further ELOC draws for the next 30 trading days or until its stock reaches $10 per share, whichever comes first.
Chronic Thromboembolic Pulmonary Hypertension Drug Chapters
Marketed Chronic Thromboembolic Pulmonary Hypertension Drugs
ADEMPAS (riociguat): BAYER
This is the first and the only therapy approved in the US to treat people with CTEPH who cannot have surgery, or for people with pulmonary hypertension that continues after surgery. Riociguat is a stimulator of soluble guanylate cyclase (sGC), an enzyme in the cardiopulmonary system and the receptor for nitric oxide. When nitric oxide binds to sGC, the enzyme catalyzes the synthesis of the signaling molecule cyclic guanosine monophosphate(cGMP). Intracellular cGMP plays an important role in regulating processes that influence vascular tone, proliferation, fibrosis, and inflammation. It comes in the form of tablets with a strength ranging from 0.5 mg to 2.5 mg. The dose can be adjusted based on the patient’s condition. It was approved by the US FDA in 2013.
Note: Detailed marketed therapies assessment will be provided in the final report.
|
Drug |
MoA |
RoA |
Company |
|
ADEMPAS (Riociguat) |
Soluble guanylate cyclase (sGC) stimulator |
Oral |
BAYER |
|
XXX |
XX |
X |
XXX |
Emerging Chronic Thromboembolic Pulmonary Hypertension Drugs
The pipeline of CTEPH is quite robust with several products available in the developmental stage. There are several key players involved in the development of promising products such as Sci Pharma, and others
Treprostinil: SciPharm Sarl
Treprostinil, developed by SciPharm Sarl, is indicated for adults with chronic thromboembolic pulmonary hypertension (CTEPH) who are ineligible for surgery or whose condition persists or recurs after surgery. In February 2013, the European Commission granted orphan designation to SciPharm for treprostinil sodium for the treatment of CTEPH. A Phase III clinical trial investigating the efficacy and tolerability of subcutaneous treprostinil sodium in severe non-operable CTEPH patients has been completed.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Treprostinil |
Prostacyclin analog promoting vasodilation |
Oral |
SciPharm Sarl |
III |
|
XXX |
XX |
X |
XXX |
X |
Note: Detailed emerging therapies assessment will be provided in the final report.
Chronic Thromboembolic Pulmonary Hypertension Market Segmentation
DelveInsight’s ‘Chronic Thromboembolic Pulmonary Hypertension Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Chronic Thromboembolic Pulmonary Hypertension market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Chronic Thromboembolic Pulmonary Hypertension Market Size by Countries
The chronic thromboembolic pulmonary hypertension market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2022, the United States held a significant share of the overall 7MM (Seven Major Markets) Chronic Thromboembolic Pulmonary Hypertension market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Chronic Thromboembolic Pulmonary Hypertension Market Size by Therapies
Chronic Thromboembolic Pulmonary Hypertension Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is Treprostinil by SciPharm Sarl.
Note: Detailed market segment assessment will be provided in the final report.
Chronic Thromboembolic Pulmonary Hypertension Drug Uptake
This section focuses on the sales uptake of potential Chronic Thromboembolic Pulmonary Hypertension drugs that have recently been launched or are anticipated to be launched in the chronic thromboembolic pulmonary hypertension market between 2020 and 2034. It estimates the market penetration of Chronic Thromboembolic Pulmonary Hypertension drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the chronic thromboembolic pulmonary hypertension market.
The emerging chronic thromboembolic pulmonary hypertension therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the chronic thromboembolic pulmonary hypertension market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report
Chronic Thromboembolic Pulmonary Hypertension Market Access and Reimbursement
DelveInsight’s ‘Chronic Thromboembolic Pulmonary Hypertension – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of CTEPH.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current chronic thromboembolic pulmonary hypertension market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the chronic thromboembolic pulmonary hypertension domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns for Chronic Thromboembolic Pulmonary Hypertension (CTEPH) market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) unmet needs.
Chronic Thromboembolic Pulmonary Hypertension: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the UC San Diego Health, Brigham and Women's Hospital (BWH), and CHRU Besancon Hospital among others.
“Despite being considered a rare disease, CTEPH is often underdiagnosed, with estimates suggesting that only a fraction of cases are recognized and treated appropriately.”
“There is a pressing need for continued research and innovation in CTEPH treatment to develop novel therapies targeting various disease pathways. Patient education is paramount to enhance treatment adherence and empower individuals in managing their condition effectively.”
Note: Detailed assessment of KOL Views will be provided in the full report CTEPH.
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the CTEPH Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Chronic Thromboembolic Pulmonary Hypertension Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for CTEPH. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Chronic Thromboembolic Pulmonary Hypertension (CTEPH) therapies.
Chronic Thromboembolic Pulmonary Hypertension Report Insights
- Chronic Thromboembolic Pulmonary Hypertension Patient Population
- Therapeutic Approaches
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Pipeline Analysis
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market Size and Trends
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market Opportunities
- Impact of Upcoming Therapies
Chronic Thromboembolic Pulmonary Hypertension Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Drug Uptake
Chronic Thromboembolic Pulmonary Hypertension Report Assessment
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Current Treatment Practices
- Unmet Needs
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Pipeline Product Profiles
- Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market Attractiveness
Key Questions
- How common is Chronic Thromboembolic Pulmonary Hypertension (CTEPH)?
- What are the key findings of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Chronic Thromboembolic Pulmonary Hypertension (CTEPH)?
- What are the disease risks, burdens, and unmet needs of Chronic Thromboembolic Pulmonary Hypertension (CTEPH)?
- At what CAGR is the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) market and its epidemiology is expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2020–2034)?
- How many companies are currently developing therapies for the treatment of Chronic Thromboembolic Pulmonary Hypertension (CTEPH)?