Chronic Venous Ulceration Market Summary
Chronic Venous Ulceration Market and Epidemiology Analysis
- According to the estimates, the highest Chronic Venous Ulceration market size was found in the United States and the least was in Spain across the 7MM. The upcoming therapies for Chronic Venous Ulceration are expected to combat the current unmet needs faced by the Chronic Venous Ulceration Patients and add to the overall growth of the Chronic Venous Ulceration market size.
- Chronic Venous Ulcerationis a common manifestation of chronic venous insufficiency (CVI), with varying prevalence across different regions. Globally, it is estimated that 1-3% of the adult population may suffer from venous leg ulcers at some point in their lives.
- There is a lack of approved therapy, and the therapies are not defined as per the severity of the disease but rather it is common for all chronic cases.
- The standard treatment for healing venous leg ulcers is compression therapy consisting of a tightly wrapped bandage that produces pressure around the ulcer site and back up to the heart. Although it can be an effective treatment, compression therapy may take a long time to heal the ulcer and does not work for everyone.
- Organogenesis’s APLIGRAF, an FDA-approved treatment for venous leg ulcers and diabetic foot ulcers lasting longer than one month that have not adequately responded to conventional therapy, become the first wound healing therapy to demonstrate a significant change in chronic wound's genomic profile, converting the chronic wound profile to resemble an acute, healing wound profile.
- At present, there are not any FDA-approved Chronic Venous Ulceration medications. However, a novel cell therapy, AMESANAR, developed by RHEACELL has been granted marketing authorization in Germany to be used in patients with chronic wounds caused by chronic venous insufficiency.
- The emerging Chronic Venous Ulceration treatment landscape is majorly focused on stem cell therapy, skin grafting, or personalized tissue-engineered veins which signifies that the cost of these therapies is likely to be high.
- Overall, acute ulcers (three months or less) have a 71 to 80 percent chance of healing, whereas chronic ulcers have only a 22 percent chance of healing after six months of treatment.
Request for Unlocking the Sample Page of the "Chronic Venous Ulceration Treatment Market"
Key Factors Impacting the Chronic Venous Ulceration Market
-
Rising Prevalence of Chronic Venous Insufficiency
A growing global burden of chronic venous disorders, driven by aging populations, sedentary lifestyles, and obesity, is contributing to an increasing number of patients developing chronic venous ulcers.
-
High Rate of Recurrence and Long Healing Duration
Chronic venous ulcers often require prolonged treatment and have high recurrence rates, creating sustained demand for advanced wound care therapies and long-term management solutions.
-
Advancements in Wound Care Technologies
Innovations such as bioengineered skin substitutes, compression devices, growth factor–based therapies, and negative pressure wound systems are enhancing treatment outcomes and expanding the market.
-
Growing Adoption of Minimally Invasive Therapies
Techniques like endovenous laser therapy (EVLT), radiofrequency ablation (RFA), and sclerotherapy are gaining traction for addressing underlying venous reflux, driving treatment uptake.
-
Increased Healthcare Awareness and Early Diagnosis
Improved patient and physician awareness about chronic venous diseases and the importance of early intervention is accelerating diagnosis and broadening the treatment pool.
-
Rising Healthcare Expenditure and Access to Advanced Care
Higher spending on wound management and access to specialized wound care centers are supporting market growth across major geographies.
-
Strong Pipeline of Emerging Therapies
Multiple novel candidates focusing on wound healing modulation, angiogenesis, and anti-inflammatory pathways are expected to transform future treatment options, contributing to market expansion.
DelveInsight's “Chronic Venous Ulceration Treatment Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of Chronic Venous Ulceration epidemiology, market, and clinical development in Chronic Venous Ulceration. In addition to this, the report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the Chronic Venous Ulceration market trends in the United States, EU4 (Germany, Spain, Italy, and France) the United Kingdom, and Japan.
Chronic Venous Ulceration Treatment Market Report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted Chronic Venous Ulceration market size from 2020 to 2034 in 7MM. The report also covers current Chronic Venous Ulceration treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Scope of the Chronic Venous Ulceration Market | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan |
|
Chronic Venous Ulceration Epidemiology |
Segmented by:
|
|
Chronic Venous Ulceration Companies |
|
|
Chronic Venous Ulceration Drugs |
|
|
Chronic Venous Ulceration Market |
Segmented by:
|
|
Analysis |
|
Chronic Venous Ulceration Understanding
Chronic Venous Ulceration Overview
Venous ulcer is defined as a full-thickness defect of the skin, most frequently in the ankle region, that fails to heal spontaneously and is sustained by chronic venous disease. Chronic vein ulcer is a defect in the skin below the level of the knee that occurs due to improper functioning of venous valves, persisting for more than six weeks with no tendency to heal after three or more months. Hence, also known as leg ulcers lower limb ulcers venous ulcers, venous insufficiency, or stasis ulcers. The symptoms of a Venous Ulcer include pain, itching, and swelling in the affected area. There may also be discolored or hardened skin around the ulcer, and the sore may produce a foul-smelling discharge.
Chronic Venous Ulceration Diagnosis
On physical examination, venous ulcers are generally irregular and shallow. Initial noninvasive imaging with comprehensive venous duplex ultrasonography, arterial pulse examination, and measurement of ankle-brachial index is recommended for all patients with suspected venous ulcers. Currently, widely available duplex scanning is considered the standard test of choice. Venography and imaging with contrast CT scan or MR venography are used infrequently and usually only when there is uncertainty about the Duplex scan information. Other methods of diagnosing venous diseases such as plethysmography and handheld Doppler do not give a good indication of the sites of venous incompetence. In a Duplex scan for chronic venous disease, the deep veins, superficial veins, and the communicating veins are evaluated.
Further details related to country-based variations in diagnosis are provided in the report
Chronic Venous Ulceration Treatment
Treatment options for venous ulcers include leg elevation, compression therapy, dressings, and medications. Compression therapy is the standard of care for Chronic Venous Ulceration. Compression bandages and compression stockings are most commonly used for compression therapy. Medications for Chronic Venous Ulceration include pentoxifylline, aspirin, and simvastatin along with other antibiotics, antiseptics, and anti-inflammatory drugs. Surgical management may be considered for ulcers that are large, of prolonged duration, or refractory to conservative measures. Surgery to the leg veins in patients with venous ulcers is most commonly used to reduce the risk of ulcer recurrence. Additionally, skin grafts are sometimes used to stimulate healing.
The Chronic Venous Ulceration treatment market report provides an overview of Chronic Venous Ulceration pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
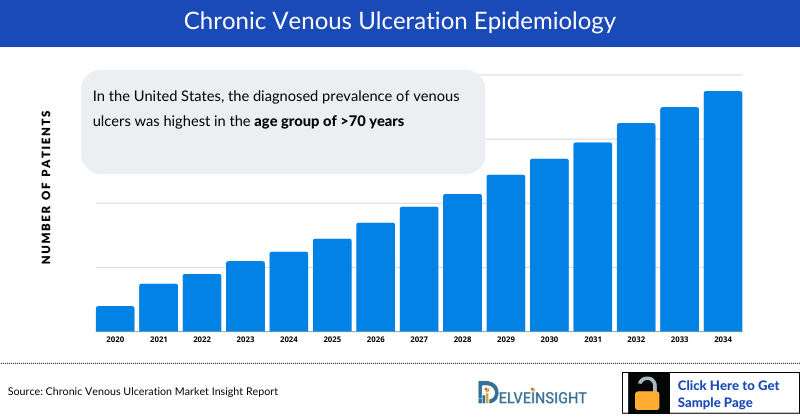
Chronic Venous Ulceration Epidemiology
The Chronic Venous Ulceration epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total prevalent cases of venous ulcers, diagnosed prevalent cases of venous ulcers, diagnosed prevalent cases of venous leg ulcers, type-specific cases of venous ulcers, gender-specific cases of venous ulcers, and age-specific cases of venous ulcers in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
Key findings from Chronic Venous Ulceration Epidemiological Analysis and Forecast
- In 2023, the United States accounted for the highest Chronic Venous Ulceration Prevalence of venous ulcers.
- In EU4 and the UK, the total Chronic Venous Ulceration Diagnosed Prevalent Population was observed to be maximum in Germany followed by France. While the least number of cases were observed in Spain in 2023.
- Chronic venous insufficiency is the cause of nearly 80% of lower leg ulcers.
- In the United States, the diagnosed prevalence of venous ulcers was highest in the age group of >70 years
- As per the estimates, it has been observed that venous ulcers are more common in females as compared to males.
Chronic Venous Ulceration Epidemiology Segmentation in the 7MM
- Total prevalent cases of venous ulcers
- Diagnosed prevalent cases of venous ulcers
- Diagnosed prevalent cases of venous leg ulcers
- Type-specific cases of venous ulcers
- Gender-specific cases of venous ulcers
- Age-specific cases of venous ulcers
Chronic Venous Ulceration Drug Analysis
The drug chapter segment of the Chronic Venous Ulceration therapeutics market report encloses a detailed analysis of Chronic Venous Ulceration marketed drugs and late-stage (Phase III and Phase II) Chronic Venous Ulceration Pipeline Drugs. It also deep dives into the Chronic Venous Ulceration clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Chronic Venous Ulceration Marketed Drugs
-
AMESANAR (allogeneic ABCB5-positive mesenchymal stromal cells/Allo-APZ2-CVU): RHEACELL
Allogeneic ABCB5-positive Stem Cells/ Allo-APZ2-CVU developed by Rheacell, a subsidiary of Ticeba, for the treatment of Chronic Venous Ulcers. Exerting immunomodulatory and anti-inflammatory effects involving several pathways, human dermal ABCB5+ MSCs/ Allo-APZ2-CVU can be reliably isolated, expanded, and manufactured as a homogenous MSC population for future in-human use as an advanced-therapy medicinal product (ATMP). In October 2021, AMESANAR was granted national approval under section 4b of the German Medicinal Products Act to be used in patients with chronic wounds caused by chronic venous insufficiency. In December 2021, the product was listed on the website of Germany's Federal Institute for Vaccines and Biomedicines.
Currently, the company is conducting a Phase III (NCT06489028) trial to investigate the efficacy and safety of allo-APZ2-CVU, administered topically on therapy-resistant non-healing CVUs, and another Phase II (NCT04971161) trial for the treatment of CVU.
Chronic Venous Ulceration Emerging Drugs
-
EscharEx: MediWound
MediWound’s EscharEx is a topical biological drug candidate for the debridement of chronic and other hard-to-heal wounds using the same proteolytic enzyme technology as NEXOBRID. It is a mixture of proteolytic enzymes for the management of the debridement of chronic and other hard-to-heal wounds. It is easy to use, and it is a non-surgical topical application that demonstrated safety and efficacy in the debriding of chronic wounds in several etiologies within a few daily applications. Recently Phase II clinical study of EscharEx for the debridement of venous leg ulcers (VLUs) also received positive results.
-
P-TEV (personalized tissue-engineered vein): VERIGRAFT
VERIGRAFT’S lead product, P-TEV is a personalized tissue-engineered vein (P-TEV) graft for use in surgical implantation to replace a defective or missing part of a patient's vein. The P-TEV drug substance consists of an extracellular matrix (ECM) scaffold in the form of a decellularized (DC) allogeneic vein scaffold which is populated with autologous components from the patient's peripheral whole blood (PWB) in an ATMP manufacturing process performed under GMP. The company is currently evaluating the product in a Phase I (NCT03784131) trial in patients with severe chronic venous insufficiency.
|
Table 2: Comparison of key emerging drugs | |||
|
Drug name |
Company |
RoA |
Phase |
|
EscharEx |
MediWound |
Topical |
III (for VLU patients planned to begin in the second half of 2024) |
|
P-TEV |
VERIGRAFT |
Implant |
I |
Chronic Venous Ulceration Market Outlook
The current treatment modalities for venous ulcers include conservative management, mechanical modalities, medications, advanced wound therapy, and surgical options. Although the main goal of treatment is ulcer healing, secondary goals include reducing edema and preventing recurrence. Evidence-based treatment options for venous ulcers include leg elevation, compression therapy, dressings, pentoxifylline, and aspirin therapy. Topical antiseptics, including cadexomer iodine (Iodosorb), povidone-iodine (Betadine), peroxide-based preparations, honey-based preparations, and silver, have also been used to treat venous ulcers. Surgical management may be considered for ulcers that are large, of prolonged duration, or refractory to conservative measures. AMESANAR is the only product that has been granted national approval under section 4b of the German Medicinal Products Act to be used in patients with chronic wounds caused by chronic venous insufficiency.
- In 2023, the United States accounts for the largest Chronic Venous Ulceration market size, in comparison to EU4 and the UK (Germany, France, Italy, the UK, and Spain) and Japan.
- Among the EU4 and the UK, Germany had the highest Chronic Venous Ulceration market size, while Spain had the lowest Chronic Venous Ulceration market size in 2023.
- The emerging landscape for Chronic Venous Ulceration is not very crowded with only a few treatment options such as EscharEx, P-TEV, and others being evaluated in the pipeline. The expected launch of these therapies may increase the Chronic Venous Ulceration market size in the coming years.
Chronic Venous Ulceration Drugs Uptake
The Phase II study of EscharEx successfully met its primary endpoint, demonstrating significant results including increased rates of complete debridement and faster achievement of debridement compared to standard hydrogel treatment. This has generated considerable anticipation regarding the potential approval and adoption of ESCHAREX. Additionally, the positive outcomes from the Phase II pharmacology study conducted in the United States have strengthened confidence in the effectiveness and safety of this innovative treatment approach. These compelling findings are expected to lead to greater acceptance of EscharEx among healthcare professionals seeking better solutions for managing chronic venous ulcers, potentially enhancing patient outcomes and quality of life for those suffering from this challenging condition. Phase III study of EscharEx for treating venous leg ulcers is scheduled to start in the second half of 2024.
This section focuses on the uptake rate of potential Chronic Venous Ulceration drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Chronic Venous Ulceration Clinical Trials Activities
The Chronic Venous Ulceration Therapeutics Market Report provides insights into different therapeutic candidates in the Phase III, Phase II, and Phase I stages. It also analyzes key Chronic Venous Ulceration Companies involved in developing targeted therapeutics.
Chronic Venous Ulceration Pipeline Development Activities
The Chronic Venous Ulceration market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Chronic Venous Ulceration emerging therapies.
Latest KOL Views on Chronic Venous Ulceration
To keep up with the real-world scenario in current and emerging Chronic Venous Ulceration market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Professors, and Others.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as the Vascular Institute of New York, Carolina Vein Institute, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Chronic Venous Ulceration market trends.
|
KOL Views |
|
“If using compression bandages or stockings, people with venous leg ulcers probably experience complete wound healing more quickly, and more people have wounds completely healed. The use of compression bandages or stockings probably reduces pain and may improve disease‐specific quality of life. There is uncertainty about adverse effects and cost-effectiveness.” |
Chronic Venous Ulceration Qualitative Analysis Report
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. Conjoint Analysis analyzes multiple approved and emerging Chronic Venous Ulceration therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Chronic Venous Ulceration Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Chronic Venous Ulceration Market Report Scope
- The Chronic Venous Ulceration treatment market report covers a segment of key events, an executive summary, descriptive overview of Chronic Venous Ulceration, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging Chronic Venous Ulceration therapies, along with the elaborative profiles of prominent therapies, will have an impact on the current Chronic Venous Ulceration Treatment Market landscape.
- A detailed review of the Chronic Venous Ulceration Market, historical and forecasted Chronic Venous Ulceration Market Size, Chronic Venous Ulceration Market Share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Chronic Venous Ulceration Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Chronic Venous Ulceration market.
Chronic Venous Ulceration Market Report Insights
- Patient-based Chronic Venous Ulceration Market Forecasting
- Therapeutic Approaches
- Chronic Venous Ulceration Pipeline Analysis
- Chronic Venous Ulceration Market Size
- Chronic Venous Ulceration Drugs Market Trends
- Existing and Future Chronic Venous Ulceration Market Opportunity
Chronic Venous Ulceration Market Report Key Strengths
- 11 Years Chronic Venous Ulceration Market Forecast
- 7MM Coverage
- Chronic Venous Ulceration Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Chronic Venous Ulceration Drugs Uptake
- Key Chronic Venous Ulceration Market Forecast Assumptions
Chronic Venous Ulceration Market Report Assessment
- Current Chronic Venous Ulceration Treatment Practices
- Chronic Venous Ulceration Unmet Needs
- Chronic Venous Ulceration Pipeline Drugs Profiles
- Chronic Venous Ulceration Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered in the Chronic Venous Ulceration Market Report
Chronic Venous Ulceration Market Insights
- What is the historical and forecasted Chronic Venous Ulceration patient pool/patient burden in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What was the Chronic Venous Ulceration market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Which treatment approaches will have a significant impact on the Chronic Venous Ulceration market size?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of Chronic Venous Ulceration?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy the Chronic Venous Ulceration Market Report
- The Chronic Venous Ulceration market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Chronic Venous Ulceration Market.
- Insights on patient burden/disease Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Chronic Venous Ulceration market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Patient-based forecast model which uses bottom-up forecasting techniques is accepted as a gold standard in pharma forecasting.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Chronic Venous Ulceration companies can strengthen their development and launch strategy.
Stay updated with us for Recent Articles @ New DelveInsight Blogs