Cognitive Impairment Associated with Schizophrenia Market
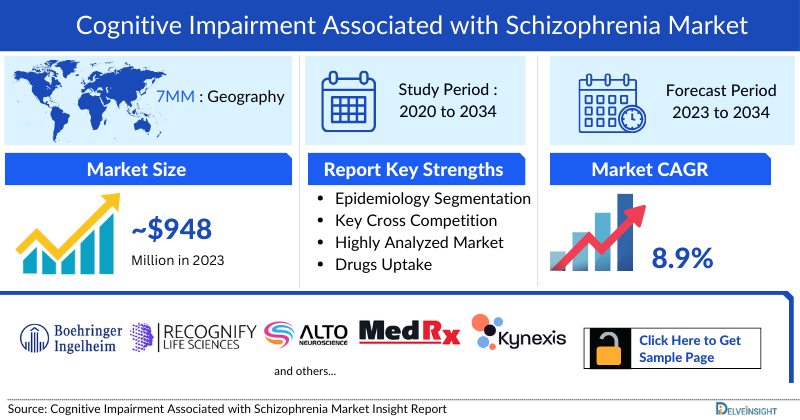
- According to DelveInsight’s analysis, the Cognitive Impairment Associated with Schizophrenia market in the 7MM was valued at approximately USD 948.1 million in 2023. Over the forecast period from 2024 to 2034, this market is projected to grow at a CAGR of 8.9%.
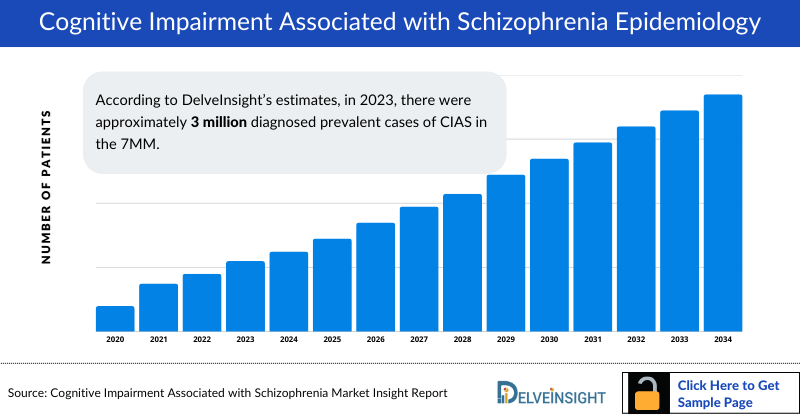
- According to DelveInsight’s estimates, in 2023, there were approximately 3 million diagnosed Cognitive Impairment Associated with Schizophrenia prevalent cases in the 7MM. Of these, the United States accounted for 52% of the cases, while EU4 and the UK accounted for nearly 33% and Japan represented 15% of the cases, respectively.
- The Cognitive Impairment Associated with Schizophrenia market is expected to experience steady growth, with a strong compound annual growth rate (CAGR) forecasted from 2024 to 2034. This growth across the 7MM will be propelled by the introduction of innovative Cognitive Impairment Associated With Schizophrenia therapies such as iclepertin (BI-425809), ALTO-101 and RL-007. Furthermore, the rising prevalence of CIAS, driven by increasing awareness and risk factors like neurobiological abnormalities and genetic and family history, will contribute to the market's expansion.
- The Cognitive Impairment Associated with Schizophrenia treatment market remains underdeveloped, with no FDA-approved Cognitive Impairment Associated With Schizophrenia treatments, with current Cognitive Impairment Associated With Schizophrenia therapies offering limited symptom relief and significant side effects. There is a critical need for targeted Cognitive Impairment Associated With Schizophrenia therapies to improve outcomes and reduce recurrence.
- Boehringer Ingelheim, Recognify Life Sciences (Atai Life Sciences), and Alto Neuroscience/MEDRx are advancing their assets through various stages of development, driving innovation in the Cognitive Impairment Associated with Schizophrenia market. Their efforts are shaping a dynamic landscape and opening up significant opportunities for market growth and expansion.
- Boehringer Ingelheim’s iclepertin (BI-425809), an innovative therapy in advanced development, is in Phase III clinical trials and is anticipated to launch by 2026 for the Cognitive Impairment Associated with Schizophrenia treatment.
DelveInsight’s “Cognitive Impairment Associated With Schizophrenia (CIAS)– Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of CIAS, historical and forecasted epidemiology, as well as the CIAS market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The CIAS market report provides current treatment practices, emerging Cognitive Impairment Associated With Schizophrenia drugs, market share of individual Cognitive Impairment Associated With Schizophrenia therapies, and current and forecasted 7MM CIAS market size from 2020 to 2034. The report also covers CIAS treatment practices and unmet medical needs to curate the best opportunities and assess the market’s potential.
Key Factors Driving the Cognitive Impairment Associated with Schizophrenia Market
Rising Prevalence of CIAS
In 2023, the 7MM had ~3 million diagnosed prevalent cases of CIAS, which are further expected to rise by 2034. The rising prevalence of cognitive impairment in schizophrenia is driven by improved diagnostic practices, greater awareness, and enhanced recognition of cognitive symptoms. As healthcare professionals focus more on these aspects, earlier detection becomes more common.
Potential of Modafinil, Donepezil, and Ketamine in CIAS
Drugs already approved for other CNS disorders, such as modafinil, donepezil, and ketamine-based therapies, could be repurposed for CIAS, potentially shortening development timelines.
Advancements in Digital Cognitive Testing
The development of digital cognitive testing tools, such as computerized cognitive batteries and ecological momentary assessments (EMA), could improve real-time, objective measurement of cognitive function, facilitating remote trials.
Launch of Emerging CIAS Therapies
The pipeline features several CIAS drugs, including RL-007 from Recognify Life Sciences (Atai Life Sciences), and ALTO-101 from Alto Neuroscience/MEDRx, among others.
Cognitive Impairment Associated With Schizophrenia Understanding and Treatment Algorithm
Cognitive Impairment Associated With Schizophrenia (CIAS) is a critical aspect of the disorder, characterized by significant deficits across multiple cognitive domains such as reasoning, problem-solving, social cognition, verbal and visual memory, working memory, attention, and processing speed. These impairments often precede the onset of overt psychosis and tend to remain stable over the course of the illness in most patients.
The underlying causes of CIAS are multifactorial, influenced by a combination of genetic, neurobiological, and environmental factors. Genetic predispositions, particularly polygenic influences on neurodevelopment and synaptic function, play a pivotal role, while environmental factors such as prenatal complications, urban living conditions, substance use, and socioeconomic challenges further contribute to the severity of cognitive deficits.
Early symptoms of CIAS may include reduced motivation, emotional flatness, social withdrawal, and unusual behavior, which can progressively lead to marked cognitive challenges that significantly affect an individual’s daily life and overall functionality. These insights highlight the complex interplay of factors driving CIAS and underscore the critical need for targeted therapeutic interventions.
Cognitive Impairment Associated With Schizophrenia diagnosis
CIAS can be diagnosed using by different tests, a clinical and cognitive assessment, and a detailed medical and psychiatric history. Cognitive deficits are screened using tools like the Montreal Cognitive Assessment (MoCA) or Brief Assessment of Cognition in Schizophrenia (BACS). For a more thorough evaluation, the MATRICS Consensus Cognitive Battery (MCCB) assesses neurocognitive and social cognitive domains. Regular follow-up assessments are crucial to track cognitive changes and Cognitive Impairment Associated With Schizophrenia treatment responses.
Further details related to country-based variations are provided in the report…
Cognitive Impairment Associated With Schizophrenia Treatment
Currently, there are no approved therapies for treating CIAS. Although, Cognitive Impairment Associated With Schizophrenia treatment approaches include both pharmacological and non-pharmacological methods. Second-generation antipsychotics, such as clozapine, olanzapine, and risperidone, are favored over first-generation options due to their comparatively better, albeit modest, impact on cognitive outcomes. While these medications primarily address psychotic symptoms and offer limited cognitive benefits, no treatment is specifically approved for cognitive deficits. Non-pharmacological methods, including cognitive remediation therapy and physical exercise programs, have demonstrated effectiveness in improving cognitive function and overall quality of life, though their adoption remains inconsistent across clinical settings.
Cognitive Impairment Associated With Schizophrenia Epidemiology
As the market is derived using a patient-based model, the Cognitive Impairment Associated with Schizophrenia epidemiology chapter in the report provides historical as well as forecasted Cognitive Impairment Associated with Schizophrenia epidemiology segmented by total Cognitive Impairment Associated with Schizophrenia prevalent cases, total diagnosed prevalent cases of schizophrenia, total diagnosed Cognitive Impairment Associated With Schizophrenia prevalence, gender-specific diagnosed prevalent cases of CIAS in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In 2023, there were an estimated 3 million total diagnosed Cognitive Impairment Associated with Schizophrenia prevalent cases across the 7MM, with the number expected to rise by 2034.
- In 2023, the US recorded approximately 2.7 million prevalent cases of schizophrenia, a figure projected to grow by 2034.
- According to DelveInsight’s estimates, there were approximately 1.5 million diagnosed prevalent cases of CIAS across the US in 2023, with the number expected to rise by 2034.
- In 2023, the US reported approximately 1.3 million male and 667 thousand female cases of schizophrenia, with these numbers expected to increase by 2034.
- According to DelveInsight’s estimates, there were approximately 1.7 million diagnosed prevalent cases of schizophrenia across EU4 and the UK in 2023, with the number expected to rise by 2034.
- In 2023, there were approximately 1.0 million male and 652 thousand female cases of schizophrenia across the EU4 and the UK.
- According to DelveInsight’s estimates, there were approximately 455 thousand diagnosed prevalent cases of CIAS across the Japan in 2023, with the number expected to rise by 2034.
Cognitive Impairment Associated With Schizophrenia Drug Chapters
The drug chapter segment of the Cognitive Impairment Associated with Schizophrenia market report encloses a detailed analysis of Cognitive Impairment Associated With Schizophrenia marketed drugs and mid to late-stage (Phase III and Phase II) cogntive impairment associated with schizophrenia pipeline drugs. It also helps understand the CIAS clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Cognitive Impairment Associated With Schizophrenia Emerging Drugs
Iclepertin (BI-425809): Boehringer Ingelheim
Iclepertin (BI-425809), developed by Boehringer Ingelheim, is an investigational oral Glycine Transporter 1 (GlyT1) inhibitor aimed at treating CIAS. By modulating glycine levels in the synaptic cleft, it enhances NMDA receptor function, potentially improving synaptic plasticity and cognitive performance. Recognizing its promise, the US FDA granted Breakthrough Therapy Designation (BTD) to iclepertin in May 2021 for the Cognitive Impairment Associated With Schizophrenia treatment, marking it as a significant step forward in addressing cognitive deficits in mental health disorders.
Iclepertin demonstrated significant cognitive benefits in schizophrenia patients during a Phase II Cognitive Impairment Associated With Schizophrenia clinical trials, with findings presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress in 2020. It is currently in Phase III evaluation as part of the CONNEX-3 program.
RL-007: Recognify Life Sciences (Atai Life Sciences)
RL-007, developed by Recognify Life Sciences, a subsidiary of Atai Life Sciences, is an innovative small molecule targeting CIAS. It enhances synaptic plasticity by modulating excitatory neurotransmission and GABA-B receptor systems, improving cognitive functions like memory and executive skills. Positive Phase IIa results in 2021 underscored its pro-cognitive potential and safety profile. It is now in a Phase IIb proof-of-concept trial, with outcomes anticipated by mid-2025.
ALTO-101: Alto Neuroscience/MEDRx
ALTO-101 is an innovative small molecule developed by Alto Neuroscience, in collaboration with MEDRx, for treating Cognitive Impairment Associated with Schizophrenia (CIAS). As a brain-penetrant PDE4 inhibitor, it targets cAMP, a molecule critical for cognition and neuroplasticity, and uses a Transdermal Delivery System (TDS) to minimize side effects while enhancing therapeutic efficacy.
Phase I trials in April 2024 demonstrated improved pharmacokinetics and tolerability with the transdermal formulation, leading to a Phase II trial initiated in June 2024, with results expected by second half of 2025. Alto has also highlighted ALTO-101’s potential at key psychiatric conferences and entered a development agreement with MEDRx, including milestone payments tied to clinical progress.
Cognitive Impairment Associated With Schizophrenia Drug Class Insights
Currently, there are no approved Cognitive Impairment Associated with Schizophrenia therapies specifically for Cognitive Impairment Associated with Schizophrenia, advances in translational biomarkers are supporting clinical development. Ongoing research focuses on neurocognitive patterns in schizophrenia, with particular attention to glutamatergic and dopamine theories, as well as progress in biomarker-based strategies for drug discovery.
Antipsychotic medications are a cornerstone of Cognitive Impairment Associated with Schizophrenia treatment, helping to manage psychotic symptoms and clinical stabilization. Second-generation Antipsychotics (SGAs) have shown small cognitive benefits compared to first-generation drugs, and long-acting injectable formulations reduce hospitalizations and relapse but do not significantly improve cognition. Newer SGAs like lurasidone, cariprazine, brexpiprazole, and lumateperone, which function as partial dopamine receptor agonists, may offer improved cognitive profiles, though further research is needed. Clozapine, while not universally beneficial for cognition, can show minimal improvement in individuals with Treatment-resistant Schizophrenia (TRS).
Continued in report…
Cognitive Impairment Associated With Schizophrenia Market Outlook
Cognitive impairment is a key feature of schizophrenia, affecting attention, memory, executive functioning, and social cognition and significantly impacting daily functioning and quality of life. These deficits are linked to neurobiological changes in the brain, particularly in the prefrontal cortex. Notably, cognitive symptoms can appear before psychosis, indicating they are intrinsic to the disorder rather than side effects of antipsychotic medications. These impairments are crucial in determining functional outcomes for patients.
Currently, no therapies are specifically approved for CIAS, and treatment typically involves off- both pharmacological and non-pharmacological methods. While second-generation antipsychotics offer modest cognitive improvements, newer agents like lurasidone and cariprazine are being explored. Adjunctive treatments such as buspirone and memantine show mixed results. Cognitive remediation therapy (CRT) is effective, and strategies like physical exercise and transcranial direct current stimulation (tDCS) provide additional benefits. However, more effective, targeted therapies are needed to address cognitive deficits in schizophrenia.
The development pipeline for Cognitive Impairment Associated with Schizophrenia treatment is limited, with iclepertin, RL-007, and ALTO-101 being the primary candidates. There is a significant unmet need for targeted Cognitive Impairment Associated with Schizophrenia treatments, as current therapies focus on symptom management rather than addressing the underlying condition.
- The total Cognitive Impairment Associated with Schizophrenia market size in the 7MM was approximately USD 948.1 million in 2023 and is projected to increase during the forecast period (2024–2034).
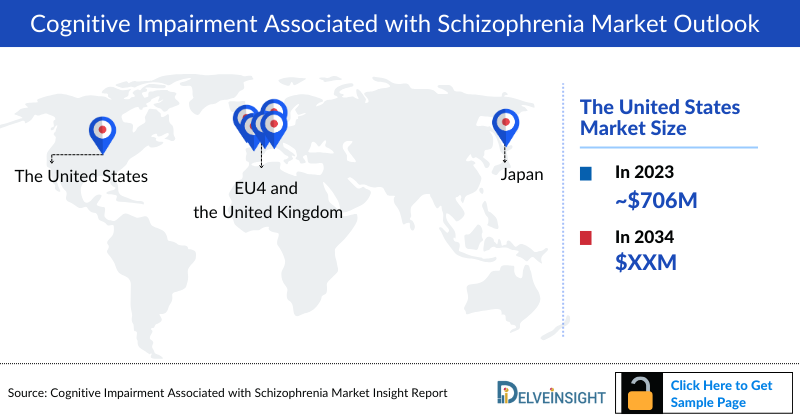
- The Cognitive Impairment Associated with Schizophrenia market size in the US was approximately USD 706.2 million in 2023 and is anticipated to increase due to the launch of emerging therapies.
- The total Cognitive Impairment Associated with Schizophrenia market size of EU4 and the UK was calculated to be approximately USD 180.5 million in 2023, which was nearly 19% of the total market revenue for the 7MM.
- In 2023, Germany led the market among the EU4 countries and the UK, generating approximately USD 49.4 million, followed by France and the UK, both contributing around USD 83.7 million.
- In 2023, the total Cognitive Impairment Associated with Schizophrenia market size was approximately USD 61.4 million in Japan which is anticipated to increase during the forecast period (2024-2034).
- Estimates suggest that iclepertin (BI-425809) is expected to generate approximately USD 933.4 million by 2034 in the 7MM.
Cognitive Impairment Associated With Schizophrenia Drugs Uptake
This section focuses on the uptake rate of potential Cognitive Impairment Associated With Schizophrenia drugs expected to be launched in the market during 2020–2034.
Further detailed analysis of emerging therapies drug uptake in the report…
Cognitive Impairment Associated With Schizophrenia Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted Cognitive Impairment Associated With Schizophrenia therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Cognitive Impairment Associated with Schizophrenia emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on CIAS evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the The Zucker Hillside Hospital, US, Columbia University Medical Center, US, Charité–Universitätsmedizin Berlin, Germany, Universite´ Franc¸ois-Rabelais, France, University of Bari Aldo Moro, Italy, University Hospital Reina Sofía, Spain, Adelphi Values, UK, and Tokushima University, Japan, among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or CIAS market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
As per the KOLs from the US, schizophrenia is a chronic mental health disorder, typically diagnosed in men during their late teens to early 20s and women in their late 20s to early 30s. It affects about 1% of the adult population in the US, translating to roughly 3 million individuals. Annually, around 100,000 Americans are diagnosed with schizophrenia. Despite progress in treating positive symptoms, over 80% of patients experience significant cognitive impairment, which remains without approved treatments. This cognitive decline plays a key role in functional disability, highlighting a critical unmet need in schizophrenia care.
As per the KOLs from France, schizophrenia is a severe psychiatric disorder marked by significant cognitive and emotional disruptions. It requires long-term medical treatment, which can lead to a range of physical, psychological, and social challenges, both from the illness itself and the side effects of treatment. The disorder affects 0.7–1% of the global population, with approximately 400,000–600,000 individuals in France. Additionally, schizophrenia is more prevalent in males (58.6% vs 48.7%) and is most commonly diagnosed in individuals aged 35–64 years.
As per the KOLs from Japan, antipsychotics, while essential in managing schizophrenia, remain underutilized due to significant side effects and limited efficacy. A promising strategy to enhance their potential lies in the application of chemical genomics, which focuses on minimizing off-target receptor activity and improving receptor specificity. By refining the chemical structures of small molecule candidates, this approach aims to optimize therapeutic efficacy while reducing adverse effects, offering new possibilities for addressing the substantial unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging Cognitive Impairment Associated With Schizophrenia therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of the Cognitive Impairment Associated With Schizophrenia therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
DelveInsight’s ‘CIAS– Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of CIAS.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
Scope of the Cognitive Impairment Associated With Schizophrenia Market Report:
- The Cognitive Impairment Associated with Schizophrenia market report covers a segment of key events, an executive summary, and a descriptive overview of CIAS, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Cognitive Impairment Associated with Schizophrenia market, historical and forecasted Cognitive Impairment Associated with Schizophrenia market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM CIAS market.
Cognitive Impairment Associated With Schizophrenia market report insights:
- Cognitive Impairment Associated with Schizophrenia Patient Population
- Cognitive Impairment Associated with Schizophrenia Therapeutic Approaches
- CIAS Pipeline Analysis
- Cognitive Impairment Associated with Schizophrenia Market Size and Trends
- Existing and Future Market Opportunity
Cognitive Impairment Associated With Schizophrenia market report key strengths:
- 11 years Forecast
- The 7MM Coverage
- CIAS Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Market Forecast Assumptions
Cognitive Impairment Associated With Schizophrenia market report assessment:
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
Key Questions from our Cognitive Impairment Associated with Schizophrenia Market Report:
Market Insights
- What was the total Cognitive Impairment Associated With Schizophrenia market size, the Cognitive Impairment Associated With Schizophrenia market size by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will iclepertin (BI-425809) affect the treatment paradigm of CIAS?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of CIAS? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to CIAS?
- What is the historical and forecasted Cognitive Impairment Associated With Schizophrenia patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent CIAS population during the forecast period (2024–2034)?
- What factors are contributing to the growth of CIAS cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the Cognitive Impairment Associated With Schizophrenia treatment? What are the current Cognitive Impairment Associated With Schizophrenia clinical guidelines and Cognitive Impairment Associated With Schizophrenia treatment guidelines?
- How many Cognitive Impairment Associated With Schizophrenia companies are developing therapies for the Cognitive Impairment Associated With Schizophrenia treatment?
- How many emerging therapies are in the mid-stage and late stage of development for treating CIAS?
- What are the recent novel therapies, targets, Cognitive Impairment Associated With Schizophrenia mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted Cognitive Impairment Associated With Schizophrenia market?
Reasons to Buy our Cognitive Impairment Associated With Schizophrenia Market Report:
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Cognitive Impairment Associated With Schizophrenia market.
- Insights on patient burden/Cognitive Impairment Associated With Schizophrenia prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for CIAS, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Get insights into our blogs:

-pipeline.png&w=256&q=75)


