Community-Acquired Bacterial Pneumonia Market
- The Community Acquired Bacterial Pneumonia Cases have increased, due to improvements in disease diagnosis and increased awareness. Further, the growing population along with lifestyle choices like lack of exercise, smoking, and drug and alcohol abuse, increasing bacterial resistance, increase the risk of developing community-acquired bacterial pneumonia.
- Diagnosing community-acquired bacterial pneumonia poses several challenges due to its diverse clinical presentation, as it makes it challenging to differentiate from other respiratory infections or non-infectious causes
- Although most community-acquired bacterial pneumonia patients are treated empirically, the best treatment choice remains unclear. The appropriate choice of antibiotic treatment is challenging to determine because the epidemiology of community-acquired bacterial pneumonia varies due to antimicrobial resistance, and the causative community-acquired bacterial pneumonia pathogens differ between countries and regions. Moreover, treatment of community-acquired bacterial pneumonia has become challenging with the changing safety profiles and efficacy of well-established antibiotics, as well as limited new therapeutic options.
- Antibiotics have been approved, which will expand the treatment options for community-acquired bacterial pneumonia, particularly in patients with primary complications. Common antimicrobials used to treat community-acquired bacterial pneumonia include macrolides (alone or in combination with β-lactams), amoxicillin (alone or combined with a macrolide), fluoroquinolones, and third-generation cephalosporins combined with a macrolide.
- Despite important advancements in the prevention of the disease through vaccines, rapid diagnostic tests, and antibiotics, community-acquired bacterial pneumonia management poses significant drawbacks.
- Early and rapid initiation of empirical antimicrobial treatment is critical for achieving favorable outcomes in community-acquired bacterial pneumonia. Initial antimicrobial therapy ought to consist of an intravenous β-lactam antibiotic plus a macrolide or a respiratory fluoroquinolone. Modification of this regimen is considered in the presence of any co-morbid conditions and risk factors for specific pathogens, but the emergence of resistant pathogens in community-acquired bacterial pneumonia continually increases the challenge for appropriate management.
- Various Community Acquired Bacterial Pneumonia Therapies like CAL02 by Eagle Pharmaceutical and Combioxin are being developed, as antibiotics alone, unfortunately, cannot win the war against pneumonia. CAL02 would serve as an add-on to standard-of-care antibiotic therapy for the prompt treatment of severe bacterial pneumonia and its devastating consequences. In an era of increasing resistance to standard therapies.
Request for unlocking the sample page of the "Community Acquired Bacterial Pneumonia Treatment Market"
DelveInsight’s “Community-Acquired Bacterial Pneumonia Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of community-acquired bacterial pneumonia, historical and forecasted epidemiology, as well as the community-acquired bacterial pneumonia market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Community Acquired Bacterial Pneumonia Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM community-acquired bacterial pneumonia market size from 2020 to 2034. The Community Acquired Bacterial Pneumonia Treatment Market Report also covers community-acquired bacterial pneumonia treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Community Acquired Bacterial Pneumonia Treatment Market: Understanding and Algorithm
Community-acquired Bacterial Pneumonia is a common, acute, severe infection of the lung parenchyma. It is a major cause of mortality in adults. It is one of the most frequent respiratory illnesses among various infections triggering sepsis. The Global Burden of Disease Study identified lower respiratory tract infection (LRTI) as the second most common cause of death and years of life lost. Microbiologically, bacteria are common agents in pneumonia, with Streptococcus pneumoniae being the most common cause worldwide.
Streptococcus pneumoniae is a bacteria most often responsible for Community-acquired pneumonia in adults worldwide. Some other common bacteria that cause community-acquired pneumonia are Haemophilus influenza and Mycoplasma pneumoniae. Pneumonia caused by chlamydia and mycoplasma is often clinically indistinguishable from other pneumonia. Symptoms of community-acquired bacterial pneumonia include malaise, chills, rigor, fever, cough, dyspnea, and chest pain. Cough typically is productive in older children and adults and dry in infants, young children, and older adults. Dyspnea usually is mild and exertional and is rarely present at rest. Chest pain is pleuritic and is adjacent to the infected area.
Community-Acquired Bacterial Pneumonia Diagnosis
Although the diagnostic criteria for community-acquired pneumonia/community-acquired bacterial pneumonia seem relatively straightforward, making the correct diagnosis can be difficult. A thoughtful history and physical examination with close attention to the actual respiratory rate and core temperature, as well as careful interpretation of chest radiographs, are required. This caution is especially true in the elderly. The clinical diagnosis of community-acquired pneumonia is made based on respiratory symptoms such as cough, sputum production, dyspnea, chest pain, signs of fever, and hypoxemia, as well as an infiltrate on chest imaging.
The diagnosis of community-acquired bacterial pneumonia is sometimes difficult because viruses, fungi, and mycobacteria may cause pneumonia, although the main causative pathogens are bacteria. In addition, there are many non-infectious diseases in the differential diagnosis of community-acquired bacterial pneumonia, such as pulmonary edema, lung cancer, acute respiratory distress syndrome, and many interstitial lung diseases.
Further details related to country-based variations are provided in the report...
Community-Acquired Bacterial Pneumonia Treatment
The Infectious Diseases Society of America and the American Thoracic Society guidelines recommend different regimens for the treatment of community-acquired bacterial pneumonia based on the patient's risk factors and local resistance patterns. For outpatient treatment of previously healthy adults without risk factors for resistant S. pneumoniae, macrolides or doxycycline are recommended. For patients with comorbidities, use of antibiotics in the last 3 months, or other risk factors for resistant S. pneumoniae, respiratory fluoroquinolones or a beta-lactam plus macrolide are recommended.
The duration of antibiotic therapy should be guided by a validated measure of clinical stability, and antibiotics should be continued until stability is achieved, for a total antibiotic duration of at least 5 days. Routine follow-up chest imaging is not recommended in adults with community-acquired bacterial pneumonia whose symptoms have resolved within 5 to 7 days. Newer antibiotics, such as nemonoxacin, levonadifloxacin, solithromycin, eravacycline, and lefamulin, have the potential to be more efficacious in the management of community-acquired bacterial pneumonia.
Community-Acquired Bacterial Pneumonia Epidemiology
As the market is derived using a patient-based model, the community-acquired bacterial pneumonia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by incident cases of community-acquired bacterial pneumonia, incidence of community-acquired bacterial pneumonia based on gender, incidence of community-acquired bacterial pneumonia based on severity, incidence of community-acquired bacterial pneumonia based on pathogens, and incidence of community-acquired bacterial pneumonia based on age in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
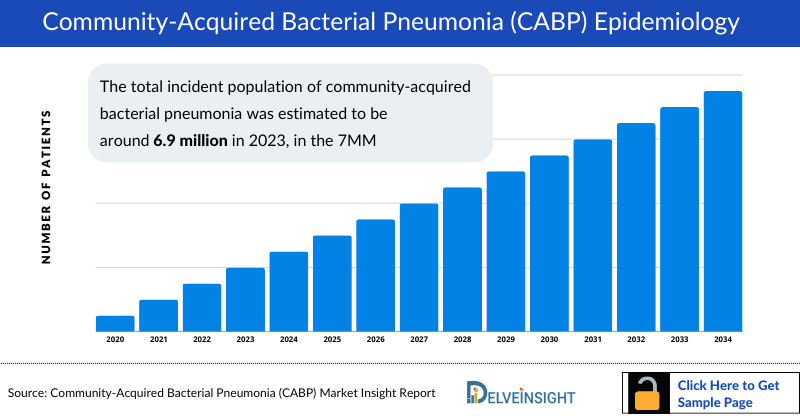
- The Community Acquired Bacterial Pneumonia Incidence is projected to increase in the next decade due to the aging population and subsequent increase in susceptibility to comorbidities. The total incident population of community-acquired bacterial pneumonia was estimated to be around 6.9 million in 2023, in the 7MM
- Of the total incident cases of community-acquired bacterial pneumonia in the 7MM, approximately 54% were found to be in the US, while EU4 and the UK accounted for 31% of the total cases, and Japan for nearly 14% of the total cases, which is projected to increase with an increase in antibiotic-resistance globally.
- The disease has a higher preponderance in males than in females and has a higher risk of worse outcomes in men than women. In 2023, there were around 57% of males and 43% of females with community-acquired bacterial pneumonia, in the US.
- The severity of community-acquired bacterial pneumonia can vary widely, ranging from mild cases characterized by fever, cough, and shortness of breath to severe cases requiring hospitalization. In EU4 and the UK, mild forms of disease accounted for the majority of community-acquired bacterial pneumonia cases, with nearly 64%, followed by moderate with 30%, and severe with 6%, in 2023. The severity of community-acquired bacterial pneumonia is a significant concern due to its association with high mortality rates and substantial morbidity.
- To address the increasing cases of community-acquired bacterial pneumonia, healthcare professionals emphasize the importance of early diagnosis and appropriate antibiotic stewardship.
- The incidence of community-acquired bacterial pneumonia varies based on the pathogen causing the infection. According to DelveInsight estimates, Streptococcus pneumonia is the most common cause of community-acquired bacterial pneumonia, accounting for 37% of the cases in the US, and 68% of the cases in EU4 and the UK. Other pathogens, such as Pseudomonas aeruginosa, Haemophilus influenzae, and Staphylococcus aureus, are also significant contributors. In Japan, Haemophilus influenzae, and Staphylococcus aureus accounted for around 19% and 18% of the total cases, in 2023.
- Age is an important risk factor for the increase in community-acquired bacterial pneumonia cases. The aging population is more susceptible to pneumonia due to weakened immune systems and underlying health conditions such as chronic lung disease, heart disease, liver cirrhosis, or diabetes. In the US, there were approximately 259 thousand, 331 thousand, 260 thousand, and 154 thousand cases of community-acquired bacterial pneumonia, for the age groups 18-49, 50-64, 65-79, and 80 and above in 2023.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Community Acquired Bacterial Pneumonia Incidence
Community-Acquired Bacterial Pneumonia Drug Chapters
The drug chapter segment of the community-acquired bacterial pneumonia drugs market report encloses a detailed analysis of community-acquired bacterial pneumonia-marketed drugs and mid to late-stage (Phase III and Phase II) Community Acquired Bacterial Pneumonia pipeline drugs. It also helps understand the community-acquired bacterial pneumonia clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Community Acquired Bacterial Pneumonia Marketed Drugs
- XENLETA (lefamulin): Nabriva Therapeutics
Lefamulin (XENLETA) is a semi-synthetic compound that inhibits the synthesis of bacterial protein, which is required for bacteria to grow. It acts by binding to the peptidyl transferase center, or PTC, on the bacterial ribosome in such a way that it interferes with the interaction of protein production at two key sites known as the “A” site and the “P” site, resulting in the inhibition of bacterial proteins and the cessation of bacterial growth. Its binding occurs with high affinity, high specificity, and at molecular sites that are different than other antibiotic classes. In 2019, the US FDA Approved XENLETA (lefamulin) for both oral and IV use.
- NUZYRA(omadacycline): Paratek Pharmaceuticals.
Omadacycline is an oral, tetracycline-like antibiotic used to treat moderate-to-severe infections, including community-acquired pneumonia and acute bacterial skin and skin structure infections caused by susceptible organisms. Oral omadacycline use has been associated with serum enzyme elevations during therapy but has not been implicated in clinically apparent acute liver injury cases.
In 2018, the US FDA approved omadacycline, a new tetracycline antibiotic, to provide a novel antibacterial treatment option for patients with CABP or ABSSSI; NUZYRA (omadacycline) for injection, for intravenous use NUZYRA (omadacycline) tablets, for oral use. Omadacycline is available in two once-daily formulations—as an IV infusion and as oral tablets
Community Acquired Bacterial Pneumonia Emerging Drugs
- CAL02: Eagle Pharmaceutical/Combioxin
CAL02 is a promising infection-controlling liposome consisting of cholesterol. Sphingomyelin will also be included for its unique anti-infection approach, which signifies that the underlying antibacterial roles of antimicrobial lipids need to be further addressed. With the global emergence of antibiotic resistance, antimicrobial lipids formulated in nano-carriers might provide a novel alternative in combatting infectious diseases. Eagle Pharmaceuticals was granted Qualified Infectious Disease Product (QIDP) and Fast-Track Designation for CAL02 by the US FDA, in 2023. It is a novel first-in-class anti-toxin drug candidate being developed for severe community-acquired bacterial pneumonia.
The company is currently in Phase II to assess the efficacy and safety of CAL02 administered intravenously in addition to standard of care in patients with severe community-acquired bacterial pneumonia. CAL02 represents a potential resistance-free empiric therapy to protect organs and prevent pro-inflammatory cascades leading to severe and fatal outcomes. Eagle believes that CAL02 is the first potential therapy engineered to neutralize a broad range of bacterial toxins for severe community-acquired bacterial pneumonia.
Community Acquired Bacterial Pneumonia Drugs Market Insights
The appropriate choice of antibiotic treatment is challenging to determine because the epidemiology of community-acquired bacterial pneumonia varies due to antimicrobial resistance, and the causative community-acquired bacterial pneumonia pathogens differ between countries and regions. Moreover, the treatment of CAP has become challenging with the changing safety profiles and efficacy of well-established antibiotics, as well as limited new therapeutic options. Common antimicrobials used to treat CAP include macrolides (alone or in combination with β-lactams), amoxicillin (alone or combined with a macrolide), fluoroquinolones, and third-generation cephalosporins combined with a macrolide.
- NUZYRA (omadacycline) is a novel antibiotic with both once-daily oral and intravenous (IV) formulations for the treatment of community-acquired bacterial pneumonia. It is specially designed to overcome tetracycline resistance and exhibits activity across a spectrum of bacteria, including gram-positive, gram-negative, atypicals, and other drug-resistant strains.
- BAXDELA (delafloxacin) is a new fluoroquinolone antibiotic available in intravenous and oral formulations. It is effective for infections caused by methicillin-resistant S. aureus (MRSA) or P. aeruginosa, in addition to other common causative pathogens, but is not more effective than existing antibiotic regimens. The antibacterial activity of delafloxacin is due to the inhibition of bacterial topoisomerase IV and DNA gyrase (topoisomerase II) enzymes, which are required for bacterial DNA replication, transcription, repair, and recombination. Delafloxacin exhibits a concentration-dependent bactericidal activity against gram-positive and gram-negative bacteria in vitro.
- XENLETA (lefamulin) is a systemic pleuromutilin antibacterial. It mainly inhibits bacterial protein synthesis through interactions with the A- and P-sites of the peptidyl transferase center (PTC) in domain V of the 23s rRNA of the 50S subunit. The binding pocket of the bacterial ribosome closes around the mutilin core for an induced fit that prevents the correct positioning of tRNA. It is bactericidal in vitro against S. pneumoniae, H. influenzae, and M. pneumoniae (including macrolide-resistant strains) and bacteriostatic against S. aureus and S. pyogenes at clinically relevant concentrations.
Community Acquired Bacterial Pneumonia Market Outlook
Community-acquired pneumonia is a leading cause of sickness and mortality globally. Although most CAP patients are treated empirically, the best therapeutic option remains unknown. The right antibiotic treatment is difficult to identify because the epidemiology varies due to antimicrobial resistance, and the bacteria that cause the disease vary by country and area. Furthermore, the shifting safety profiles and efficacy of well-established antibiotics, as well as a lack of alternative therapeutic choices, have made community-acquired bacterial pneumonia treatment difficult.
However, new medicines have been approved, expanding the treatment choices for community-acquired bacterial pneumonia, particularly in individuals with main problems. Antimicrobials used to treat community-acquired bacterial pneumonia include macrolides, amoxicillin, fluoroquinolones, and third-generation cephalosporins. Antibiotic resistance among common community respiratory pathogens has resulted in a demand for new antibiotics for community-acquired bacterial pneumonia; the World Health Organization's priority pathogen list for developing new antibiotics includes penicillin-nonsusceptible S. pneumoniae CABP has shown higher than 30% ampicillin resistance in H. influenzae isolates, as well as S. pneumoniae resistance to multiple classes of antibiotics (penicillin, 12.7%; tetracycline, 21.5%; and macrolide, 45.6%).
Some of the medications approved for the Community Acquired Bacterial Pneumonia Treatment are NUZYRA (omadacycline), BAXDELA (delafloxacin), and XENLETA (lefamulin). In addition, the drugs approved for hospital-acquired bacterial pneumonia (HABP)/ventilator-associated bacterial pneumonia (VABP) comprise RECARBRIO (Cilastatin/imipenem/relebactam), FETROJA (cefiderocol), AVYCAZ (ceftazidime-avibactam), VIBATIV (telavancin), and ZERBAXA (ceftolozane + tazobactam), among others.
Key Community Acquired Bacterial Pneumonia Companies such as Eagle Pharmaceutical and Combioxin among others are evaluating their lead candidates in different stages of clinical development. They aim to investigate their products to treat community-acquired bacterial pneumonia.
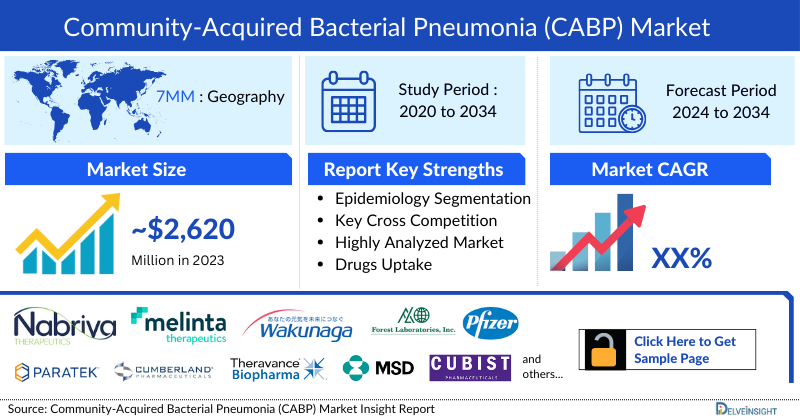
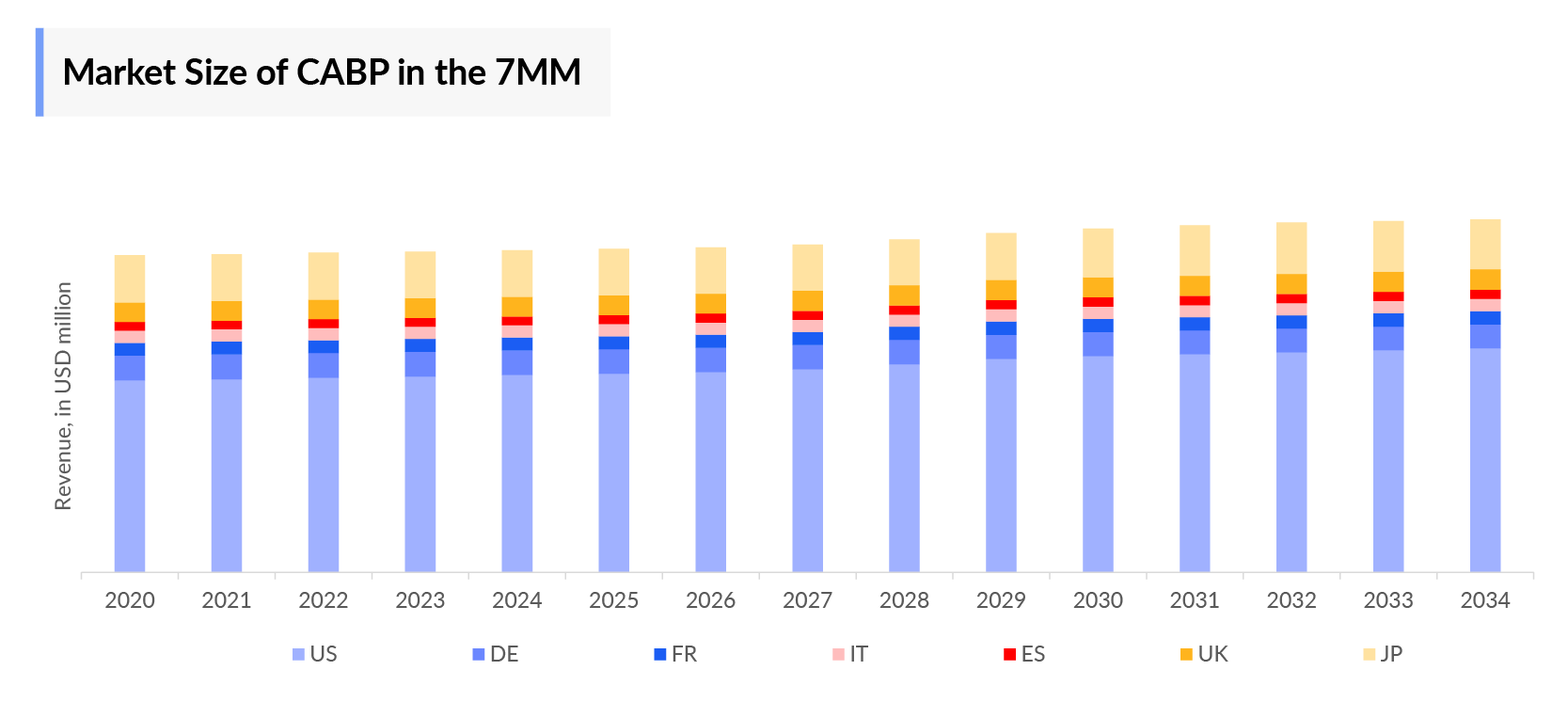
- The total Community Acquired Bacterial Pneumonia Market Size in the 7MM was ~USD 2,627 million in 2023 and is projected to increase during the forecast period (2024–2034).
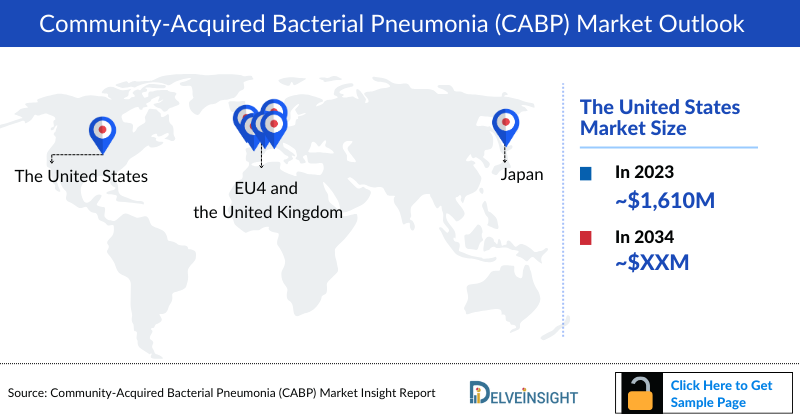
- The Community Acquired Bacterial Pneumonia Therapeutics Market in the United States was USD 1,606 million in the year 2023.
- The current Community Acquired Bacterial Pneumonia Therapeutics Market is mainly dominated by penicillins, macrolides, cephalosporins, b-lactams, tetracycline, and others. Among these broad-spectrum cephalosporin generated a revenue of USD 611.8 million, followed by tetracyclines which generated USD 423 million, respectively in 2023, in the 7MM.
- In 2023, Japan with a revenue of approximately USD 379.9 million, which is expected to increase by 2034.
- Eagle Pharmaceuticals and Combioxin’s CAL02 is the first potential therapy engineered to neutralize a broad range of bacterial toxins for severe community-acquired bacterial pneumonia. This infection-controlling liposome drug is projected to enter the US market by 2026 and is anticipated to have a medium uptake attaining its peak by 5th year.
Community-Acquired Bacterial Pneumonia Drugs Uptake
This section focuses on the uptake rate of potential Community Acquired Bacterial Pneumonia drugs expected to be launched in the market during 2020–2034. For example, Eagle Pharmaceuticals and Combioxin’s CAL02 is expected to enter the US market by 2026 and is projected to have a medium uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report….
Community-Acquired Bacterial Pneumonia Pipeline Development Activities
The Community Acquired Bacterial Pneumonia therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Community Acquired Bacterial Pneumonia Companies involved in developing targeted therapeutics.
Pipeline development activities
The Community Acquired Bacterial Pneumonia therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for Community-Acquired Bacterial Pneumonia.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Community Acquired Bacterial Pneumonia Treatment Drugs
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on community-acquired bacterial pneumonia evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the University of Washington, Johns Hopkins University School of Medicine, University Heart Centre Hamburg, Germany, Careggi University Hospital, Italy, and the Royal Brompton Hospital, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or community-acquired bacterial pneumonia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
According to our primary research analysis, the clinical and economic burden of community-acquired bacterial pneumonia is staggering, far-reaching, and expected to increase in the future as new antibiotic resistance mechanisms emerge and the world's population ages. Urgent needs in community-acquired bacterial pneumonia management include the development of new antimicrobials, adjuvant therapies, and rapid diagnostics.
Antibacterial agents used to treat common pathogens in community-acquired bacterial pneumonia are marked by adverse drug events and increasing antimicrobial resistance. Antimicrobial resistance, including in pathogens that cause community-acquired bacterial pneumonia, continues to spread at an alarming rate. Because of these factors, the development of new antibiotic classes is urgently needed.
Community Acquired Bacterial Pneumonia Therapeutics Market: Qualitative Analysis
We perform Qualitative and Community Acquired Bacterial Pneumonia Market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Community Acquired Bacterial Pneumonia Therapeutics Market Access and Reimbursement
Market access refers to the ability of all patients to have access to a given product quickly, conveniently, and affordably. Reimbursement is the price negotiation between the manufacturer and payer that allows the manufacturer access to that market. It is provided to reduce the high costs and make essential drugs affordable. Health technology assessment (HTA) refers to the systematic evaluation of properties, effects, and/or impacts of health technology. It is a multidisciplinary process to evaluate the social, economic, organizational, and ethical issues of a health intervention or health technology.
In the US healthcare system, both public and private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, the Children’s Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), third-party organizations that provide services, and educational programs to aid patients are also present.
The Community Acquired Bacterial Pneumonia Therapeutics Market report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Community Acquired Bacterial Pneumonia Treatment Market Report Scope
- The Community Acquired Bacterial Pneumonia treatment market report covers a segment of key events, an executive summary, and a descriptive overview of community-acquired bacterial pneumonia, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the community-acquired bacterial pneumonia drugs market, historical and forecasted Community Acquired Bacterial Pneumonia treatment market size, Community Acquired Bacterial Pneumonia market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Community Acquired Bacterial Pneumonia treatment market report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM community-acquired bacterial pneumonia drugs market.
Community-Acquired Bacterial Pneumonia Treatment Market Report Insights
- Patient-based Community Acquired Bacterial Pneumonia Market Forecasting
- Therapeutic Approaches
- Community-Acquired Bacterial Pneumonia Pipeline Analysis
- Community-Acquired Bacterial Pneumonia Market Size and Trends
- Existing and Future Community Acquired Bacterial Pneumonia Drugs Market Opportunity
Community-Acquired Bacterial Pneumonia Treatment Market Report Key Strengths
- 11 years Community Acquired Bacterial Pneumonia Market Forecast
- The 7MM Coverage
- Community-Acquired Bacterial Pneumonia Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Drugs Uptake and Key Community Acquired Bacterial Pneumonia Market Forecast Assumptions
Community-Acquired Bacterial Pneumonia Treatment Market Report Assessment
- Current Community Acquired Bacterial Pneumonia Treatment Market Practices
- Community Acquired Bacterial Pneumonia Unmet Needs
- Community Acquired Bacterial Pneumonia Pipeline Product Profiles
- Community Acquired Bacterial Pneumonia Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Community Acquired Bacterial Pneumonia Drugs Market Insights
- What was the total Community Acquired Bacterial Pneumonia treatment market size, the Community Acquired Bacterial Pneumonia drugs market size by therapies, and Community Acquired Bacterial Pneumonia market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will CAL02 affect the treatment paradigm of community-acquired bacterial pneumonia?
- How will CAL02 compete with other upcoming products and marketed therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the Community Acquired Bacterial Pneumonia market dynamics and subsequent analysis of the associated trends?
Community Acquired Bacterial Pneumonia Epidemiology Insights
- What are the disease risks, burdens, and Community Acquired Bacterial Pneumonia Unmet Needs? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to community-acquired bacterial pneumonia?
- What is the historical and forecasted community-acquired bacterial pneumonia patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent community-acquired bacterial pneumonia ss population during the forecast period (2024–2034)?
- What factors are contributing to the growth of community-acquired bacterial pneumonia cases?
Current Community Acquired Bacterial Pneumonia Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of community-acquired bacterial pneumonia? What are the current clinical and treatment guidelines for treating community-acquired bacterial pneumonia?
- How many Community Acquired Bacterial Pneumonia Companies are developing therapies for the treatment of community-acquired bacterial pneumonia?
- How many emerging therapies are in the mid-stage and late stage of development for treating community-acquired bacterial pneumonia?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted Community Acquired Bacterial Pneumonia Drugs Market?
Reasons to Buy
- The Community Acquired Bacterial Pneumonia Drugs Market Report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the community-acquired bacterial pneumonia therapeutics market.
- Insights on patient burden/disease Community Acquired Bacterial Pneumonia prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Community Acquired Bacterial Pneumonia drugs market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the Community Acquired Bacterial Pneumonia drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for community-acquired bacterial pneumonia, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing.
Stay Updated with us for Recent Articles
-market.png&w=256&q=75)
-02.png)
 in the 7MM-01.png)

-pipeline.png&w=256&q=75)

