Degenerative Disc Disease Market
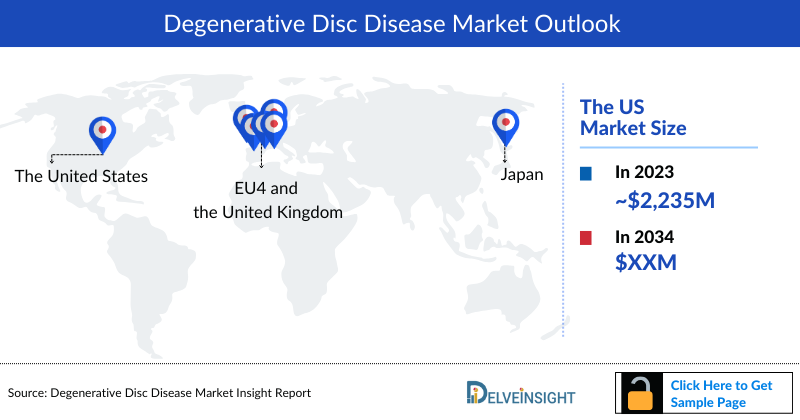
- In 2023, the market size of Degenerative Disc Disease (DDD) was highest in the US among the 7MM, accounting for approximately USD 2,235 million which is further expected to increase by 2034.
- In 2023, the diagnosed prevalence of DDD was highest in the US among the 7MM, accounting for nearly 12,919 thousand cases which is further expected to increase by 2034.
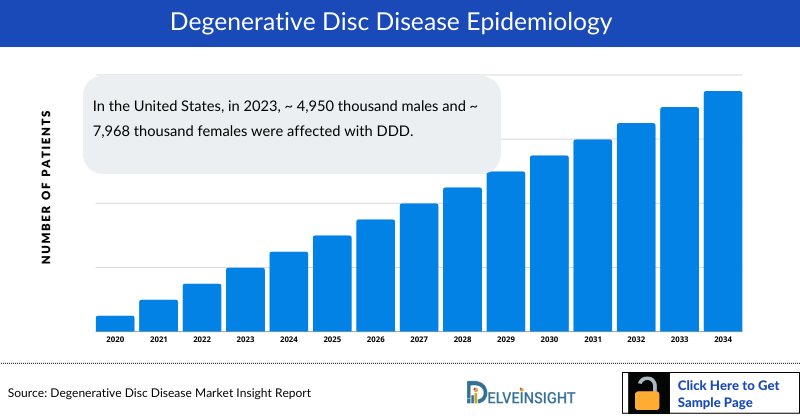
- In the United States, in 2023, ~ 4,950 thousand males and ~ 7,968 thousand females were affected with DDD.
- The current market for DDD is limited to over-the-counter treatments, including nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, acetaminophen, muscle relaxants like cyclobenzaprine, opioids, and corticosteroids. Due to the lack of approved therapies, these options offer only nonspecific and often temporary relief, making up a market size of USD 3,592 million in the 7MM in 2023. The market size is expected to increase with the projected launch of emerging therapies during the forecast period (2024–2034).
- The market size of DDD in Japan was around USD 454 million in 2023, accounting for 13% of the total market of the 7MM, which is expected to rise by 2034.
DelveInsight’s “Degenerative Disc Disease Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of the DDD, historical and forecasted epidemiology as well as the DDD market trends in the United States, EU4 and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The DDD market report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM DDD market size from 2020 to 2034. The Report also covers current DDD treatment practice, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
Degenerative Disc Disease Treatment Market
Degenerative Disc Disease (DDD) Overview
DDD, which is also known as Degenerative Disc Disease Disc degeneration is an abnormal structural failure due to the cell-mediated response to multifactorial contributions, such as genetics, micro/macro trauma, accelerated age-related changes, inflammation, local nutritional deficiency, and vascular factors, leading to excess catabolic over anabolic responses. DDD is a painful degenerated disc.
Degenerative Disc Disease (DDD) Diagnosis
The diagnosis of DDD is usually through a combination of symptoms and physical examinations. While X-rays of the spine can support the diagnosis, disc degeneration might occasionally be identified incidentally during cervical, chest, or abdominal X-rays conducted for other reasons. However, a radiologic diagnosis alone is insufficient for confirming DDD, as the radiologist may lack information about the patient's symptoms.
Current diagnostic methods, like imaging, may not necessarily align with patient symptoms, emphasizing the need for more accurate tools. Advancements in technology, such as improved imaging or biomarker identification, are essential to swiftly and precisely identify the root cause of pain in DDD. This improvement is crucial for tailoring personalized and timely interventions, ultimately enhancing patient outcomes and managing this widespread and debilitating condition.
Further details related to diagnosis are provided in the report...
Degenerative Disc Disease (DDD) Treatment
Treatment strategies for DDD focus on managing symptoms, reducing pain, and improving the patient's quality of life. These strategies are categorized into non-surgical (conservative) and surgical options, based on the severity of the condition and the patient’s needs. Non-surgical approaches encompass physical therapy to strengthen muscles supporting the spine, medications such as pain relievers and anti-inflammatories, lifestyle changes, epidural steroid injections, heat and cold therapy, and chiropractic care. When conservative methods are ineffective or the condition is severe, surgical interventions may be considered, including discectomy, spinal fusion, artificial disc replacement, intradiscal electro thermal therapy (IDET), or nucleoplasty.
Treatment selection is influenced by factors such as symptom severity, age, overall health, and patient preferences, with a general emphasis on conservative treatments before resorting to surgery. The primary goals are to alleviate baseline pain, prevent flare-ups, and manage the condition effectively through a tailored plan developed with healthcare professionals.
Further details related to treatment are provided in the report...
Degenerative Disc Disease (DDD) Epidemiology
As the market is derived using a patient-based model, the DDD epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by, Total Diagnosed Prevalent Cases of CLBP, Total Diagnosed Prevalent Cases of DDD, Gender-specific Diagnosed Prevalent Cases of DDD, and Age-specific Diagnosed Prevalent Cases of DDD in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- According to DelveInsight's assessment, the estimated number of diagnosed prevalent cases of CLBP in the US was approximately 32,297 thousand in 2023.
- In the assessment done by DelveInsight, the estimated total diagnosed prevalent cases of DDD in the 7MM were nearly 25,623 thousand in 2023.
- Among the European countries, the United Kingdom had the highest diagnosed prevalent cases of DDD with 2,121 thousand cases, followed by Germany, which had diagnosed prevalent population of ~2,033 thousand in 2023. On the other hand, Italy had the lowest prevalent population (1,042 thousand cases).
- Japan had nearly 4,289 thousand total diagnosed prevalent cases of DDD in 2023, accounting for approximately 17% in 7MM.
- Based on age-specific segmentation, the people aged 50-59 years were affected the most by DDD in the US, accounting for approximately 2,592 thousand cases in 2023.
- As per DelveInsight analysis, among the EU4 and the UK, females were affected more than males with 38% male cases and 62% female cases in 2023.
Degenerative Disc Disease (DDD) Drug Chapters
The drug chapter segment of the DDD report encloses a detailed analysis of DDD off-label drugs and late-stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the DDD clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Degenerative Disc Disease Emerging Drugs
Rexlemestrocel-L (MPC-06-ID): Mesoblast, Ltd. / Grünenthal
Mesoblast’s rexlemestrocel-L, an investigational allogeneic human bone marrow-derived mesenchymal precursor cell (MPC) therapy. MPC-06-ID is a Phase III product candidate for treating chronic low back pain caused by disc degeneration (CLBP). It is being developed for patients who have exhausted conservative treatment options, may have failed epidural steroid injections, and have no further treatment option other than invasive and costly surgical interventions.
SB-01 (Peniel2000/P2K, YH14618): Spine Biopharma/Yuhan Corp
SB-01 is a synthetic 7-amino acid peptide designed to downregulate Transforming Growth Factor Beta 1 (TGFß1), which is overexpressed in degenerated discs associated with lower back pain. By modulating TGFß1, SB-01 aims to address chronic back pain and potentially halt disc degeneration. Previously known as YH14618 and P2K, it is currently undergoing Phase III trials for lumbar DDD.
IDCT (Rebonuputemcel): DiscGenics, Inc.
IDCT (rebonuputemcel) is an investigational product that is under development by DiscGenics. IDCT is an allogeneic (donor-derived), non-invasive cell therapy being evaluated in two concurrent clinical trials in the US and Japan for treating adult patients with single-level mild to moderate symptomatic lumbar disc degeneration. During treatment, a single dose of IDCT is injected into the painful disc percutaneously (non-surgically).
In July 2024, DiscGenics, Inc. gained acceptance from the US Food and Drug Administration (FDA) for the clinical protocols and Chemistry, Manufacturing, and Controls (CMC) clinical development plan for Phase III clinical program of its allogeneic, injectable disc progenitor cell therapy (IDCT or rebonuputemcel) for painful lumbar degenerative disc disease (DDD), allowing the study to proceed.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Rexlemestrocel-L (MPC-06-ID) |
Cell Replacement |
Intradiscal injection |
Mesoblast, Ltd. / Grünenthal |
III |
|
SB-01 (Peniel2000/P2K, YH14618) |
TGFβ1 antagonists |
Intradiscal injection |
Spine BioPharma, Inc./ Yuhan Corporation |
III |
|
IDCT (Rebonuputemcel) |
Cell Replacement |
Intradiscal injection |
DiscGenics, Inc. |
XX |
|
XXX |
Cell replacements |
XXX |
XXX |
II |
Note: Detailed emerging therapies assessment will be provided in the final report of Degenerative Disc Disease (DDD)...
Degenerative Disc Disease (DDD) Market Outlook
The primary treatment goal for DDD is to manage symptoms, particularly pain, improve overall function, and prevent further degeneration of the intervertebral discs. While complete reversal of disc degeneration may not be achievable, the focus is on enhancing the individual's quality of life by minimizing pain, improving mobility, and optimizing the overall functionality of the spine. Treatment approaches often involve a combination of non-pharmacologic interventions, lifestyle modifications, and, in some cases, surgical options, depending on the severity and specific characteristics of the condition in each patient.
There is no FDA-approved DDD therapy due to its complex nature. Pharmacological interventions for DDD primarily target pain and inflammation. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) such as ibuprofen are commonly prescribed for their anti-inflammatory properties. Acetaminophen serves as an over-the-counter pain reliever. Muscle relaxants like cyclobenzaprine may alleviate associated spasms. In more severe cases, opioids and corticosteroid injections may be considered, under careful medical supervision, tailored to individual patient needs and response.
Non-pharmacological treatments for degenerative disc disease encompass physical therapy, lifestyle adjustments, and complementary therapies like acupuncture. These strategies aim to alleviate pain, enhance function, and mitigate the impact of degeneration on daily activities, offering a comprehensive approach for improved quality of life.
- The DDD market is poised for transformation with the anticipated introduction of therapies like SB-01, Rexlemestrocel-L, IDCT, and others. The approval of these therapies could significantly impact market dynamics, although their success rates remain uncertain.
- The market size of DDD in the 7MM was around USD 3,592 million in 2023, which is further anticipated to increase during the forecast period.
- The United States accounted for the highest market size of DDD approximately 62% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the European countries, Germany and the UK had the highest market size with nearly USD 228 million and USD 224 million each in 2023, while Italy had the lowest market size for DDD with USD ~ 110 million in 2023.
- With the expected launch of upcoming therapies, such as Rexlemestrocel-L, and IDCT, among others, the total market size of DDD is expected to show change in the upcoming years.
Degenerative Disc Disease (DDD) Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to launch in the market during 2020–2034. For example, IDCT (Rebonuputemcel) in the US is expected to be launched by 2029 with a peak share of 1.90%. IDCT is anticipated to take 7 years to peak with a slow-medium uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Degenerative Disc Disease (DDD) Pipeline Development Activities
The report provides insights into Degenerative Disc Disease clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for DDD emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Degenerative Disc Disease (DDD) evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from The Orthopedic Center Of St. Louis, Chesterfield, Missouri, US; Department of Neurological Surgery, New York–Presbyterian Hospital/Weill Cornell Medical Center, New York, US; La Sapienza University of Rome, Rome, Italy; Clinique du Parc, rue Emile-Combe, Castelnau-le-Lez, France; Department of Orthopedic Surgery, IBSAL-University Hospital of Salamanca, Salamanca, Spain; Department of Orthopaedic Surgery, Osaka University, Osaka, Japan; and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or DDD market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Degenerative Disc Disease Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Degenerative Disc Disease, explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the DDD market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM DDD market.
Degenerative Disc Disease (DDD) Report Insights
- Degenerative Disc Disease Patient Population
- Degenerative Disc Disease Therapeutic Approaches
- Degenerative Disc Disease Pipeline Analysis
- Degenerative Disc Disease Market Size and Trends
- Existing and Future Market Opportunity
Degenerative Disc Disease (DDD) Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- DDD Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Degenerative Disc Disease Drugs Uptake
- Key Degenerative Disc Disease Market Forecast Assumptions
Degenerative Disc Disease (DDD) Report Assessment
- Current Degenerative Disc Disease Treatment Practices
- Degenerative Disc Disease Unmet Needs
- Degenerative Disc Disease Pipeline Product Profiles
- Degenerative Disc Disease Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Degenerative Disc Disease Market Drivers
- Degenerative Disc Disease Market Barriers
Key Questions Answered In The Degenerative Disc Disease Market Report
Degenerative Disc Disease Market Insights
- What was the Degenerative Disc Disease (DDD) market share (%) distribution in 2020 and how it would look like in 2034?
- What would be the Degenerative Disc Disease (DDD) total market size as well as market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Degenerative Disc Disease (DDD) market size during the forecast period (2024–2034)?
- At what CAGR, the Degenerative Disc Disease (DDD) market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the Degenerative Disc Disease (DDD) market outlook across the 7MM during the forecast period (2024–2034)?
- What would be the Degenerative Disc Disease (DDD) market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Degenerative Disc Disease Epidemiology Insights
- What is the disease risk, burden, and unmet needs of Degenerative Disc Disease (DDD)?
- What is the historical DDD patient population in the United States, EU4 (Germany, France, Italy, Spain) and the UK, and Japan?
- What would be the forecasted patient population of DDD at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Degenerative Disc Disease?
- Out of the above-mentioned countries, which country would have the highest prevalent population of DDD during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current Degenerative Disc Disease Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of DDD along with the approved therapy?
- What are the current treatment guidelines for the treatment of DDD in the US, Europe, And Japan?
- What are the DDD marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, and efficacy, etc.?
- How many companies are developing therapies for the treatment of Degenerative Disc Disease?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Degenerative Disc Disease?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the DDD therapies?
- What are the recent therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for DDD and their status?
- What are the key designations that have been granted for the emerging therapies for Degenerative Disc Disease?
- What are the 7MM historical and forecasted market of Degenerative Disc Disease?
Reasons to Buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the DDD Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.
Frequently Asked Questions
1. What is the forecast period covered in the report?
The Degenerative Disc Disease (DDD) Epidemiology and Market Insight report for the 7MM covers the forecast period from 2024 to 2034, providing a projection of market dynamics and trends during this timeframe.
2. Who are the key players in the Degenerative Disc Disease (DDD) market?
The DDD market has several key players. The major players are Mesoblast, Ltd; Grünenthal; DiscGenics, and others which are currently developing drugs for the treatment of DDD.
3. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as incident cases, treatment costs, revenue generated, and market trends.
4. What is the key driver of the DDD market?
The increase in diagnosed prevalent cases of DDD and launch of emerging therapies are attributed to be the key drivers for increasing DDD market.
5. What is the expected impact of emerging therapies or advancements in DDD treatment on the market?
Introducing new therapies, advancements in diagnostic techniques, and innovations in treatment approaches can significantly impact the DDD treatment market. Market forecast reports may provide analysis and predictions regarding the potential impact of these developments.
6. Does the report provide insights into the competitive landscape of the market?
The market forecast report may include information on the competitive landscape, profiling key market players, their product offerings, partnerships, and strategies, and helping stakeholders understand the competitive dynamics of the DDD market.