Endometrial Ablation Devices Market Summary
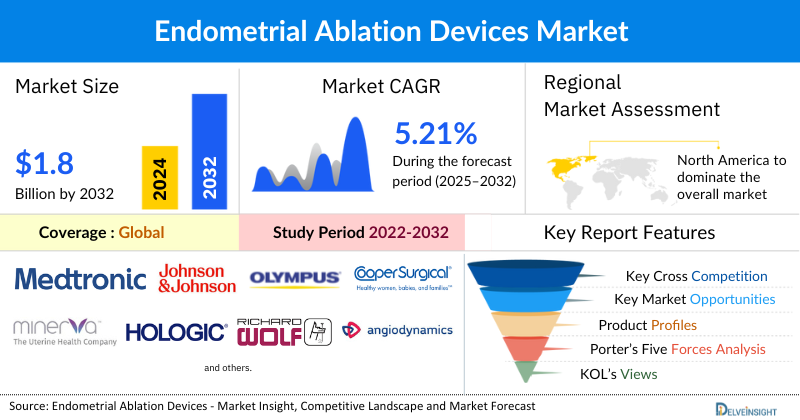
- The global endometrial ablation devices market is expected to increase from USD 1,222.96 million in 2024 to USD 1,831.90 million by 2032, reflecting strong and sustained growth.
- The global endometrial ablation devices market is growing at a CAGR of 5.21% during the forecast period from 2025 to 2032.
- The market of endometrial ablation devices is being primarily driven by the rising cases of heavy menstrual bleeding (menorrhagia) and its associated risk factors, increasing awareness about women’s health, an increase in technological advancement, growing preference for minimally invasive procedures, and an increase in product development activities among the key market players.
- The leading companies operating in the endometrial ablation devices market include Medtronic, Johnson & Johnson Services, Inc., Olympus Corporation, Minerva Surgical, Inc., Hologic, Inc., CooperSurgical Inc., Richard Wolf GmbH, AngioDynamics, KARL STORZ SE & Co. KG, IDOMAN-MED, Arthrex, Inc., Channel Medsystems, Inc., Gynesonics, Boston Scientific Corporation, AEGEA Medical, and others.
- North America is expected to dominate the endometrial ablation devices market due to the growing prevalence of abnormal uterine bleeding among women, particularly those aged 35-55. Additionally, the favorable reimbursement policies, high awareness of women's health issues, and increased product development activities across the region have further boosted the overall market of endometrial ablation devices.
- In the product type segment of the endometrial ablation devices market, the Radiofrequency (RF) ablation devices category is estimated to account for the largest market share in 2024.
Request for unlocking the report of the @ Endometrial Ablation Devices Market
Endometrial Ablation Devices Market Size and Forecasts
|
Report Metrics |
Details |
|
2024 Market Size |
USD 1,222.96 million |
|
2032 Projected Market Size |
USD 1,831.90 million |
|
Growth Rate (2025-2032) |
5.21% CAGR |
|
Largest Market |
North America |
|
Fastest Growing Market |
Asia-Pacific |
|
Market Structure |
Moderately Concentrated |
Factors Contributing to the Growth of the Endometrial Ablation Devices Market
- The rising cases of heavy menstrual bleeding (menorrhagia) and its associated risk factors: The rising cases of heavy menstrual bleeding (menorrhagia) are boosting the market for endometrial ablation devices, as more women seek effective, minimally invasive treatments to manage their symptoms. With growing awareness and diagnosis of this condition, especially among women aged 35 - 55, the demand for quick, low-risk alternatives to surgery like endometrial ablation is increasing, driving market growth.
- Increasing awareness about women’s health is escalating the demand for endometrial ablation devices: Increasing awareness about women’s health is escalating the demand for endometrial ablation devices, as more women are recognizing symptoms of conditions like heavy menstrual bleeding and seeking timely medical intervention. Health campaigns, education programs, and improved access to gynecological care are encouraging women to opt for minimally invasive treatments, thereby driving the market for these devices.
- Increase in technological advancement: Technological advancements are boosting the market for endometrial ablation devices by making procedures safer, faster, and more effective. Modern devices use advanced methods like radiofrequency, cryoablation, and thermal energy, which offer improved outcomes with minimal discomfort and quicker recovery times. These innovations are encouraging more healthcare providers and patients to adopt endometrial ablation as a preferred treatment option.
- Growing preference for minimally invasive procedures: The growing preference for minimally invasive procedures is boosting the market for endometrial ablation devices, as patients seek treatments with less pain, shorter recovery times, and lower risk compared to traditional surgery. Endometrial ablation offers a quick, outpatient solution for heavy menstrual bleeding, making it an attractive option for both patients and healthcare providers, thereby driving its demand.
Endometrial Ablation Devices Market Report Segmentation
This endometrial ablation devices market report offers a comprehensive overview of the global endometrial ablation devices market, highlighting key trends, growth drivers, challenges, and opportunities. It covers detailed market segmentation by Product Type (Radiofrequency Ablation Devices, Thermal Balloon Ablation Devices, Cryoablation Devices, Hydrothermal Ablation Devices, Microwave Ablation Devices, and Others), End-Users (Hospitals, Specialty Clinics, and Others), and Geography. The report provides valuable insights into the competitive landscape, regulatory environment, and market dynamics across major markets, including North America, Europe, and Asia-Pacific. Featuring in-depth profiles of leading industry players and recent product innovations, this report equips businesses with essential data to identify market potential, develop strategic plans, and capitalize on emerging opportunities in the rapidly growing Endometrial Ablation Devices market.
Endometrial ablation devices are specialized medical instruments used in a minimally invasive gynecological procedure to treat heavy menstrual bleeding (menorrhagia) in women who have completed childbearing. These devices are inserted through the vagina and cervix into the uterus and function by delivering energy, such as heat (from radiofrequency, heated fluid/balloon, or microwave energy) or extreme cold (cryotherapy), to intentionally destroy (ablate) the tissue lining the uterus, known as the endometrium. The destruction of this lining prevents its monthly buildup and shedding, thereby significantly reducing or stopping menstrual blood flow, and offering a less invasive alternative to a hysterectomy.
The overall market for endometrial ablation devices is experiencing significant growth due to a combination of key factors. The rising cases of heavy menstrual bleeding (menorrhagia) have led to increased demand for effective treatment options, while growing awareness about women’s health is encouraging more women to seek timely medical care. At the same time, advancements in technology have made endometrial ablation procedures safer, faster, and more efficient, enhancing their appeal among both patients and healthcare providers. The growing preference for minimally invasive procedures further supports this trend, as women increasingly opt for treatments with less pain and quicker recovery times. Additionally, increased product development activities by key market players are introducing innovative and user-friendly devices, expanding the range of options available, and driving wider adoption. Together, these factors are collectively fuelling the expansion of the endometrial ablation devices market globally.
Get More Insights into the Report @ Endometrial Ablation Devices Market
What are the latest Endometrial Ablation Devices market dynamics and trends?
The global endometrial ablation devices market is experiencing strong growth, primarily driven by the rising prevalence of heavy menstrual bleeding (HMB/menorrhagia) and related risk factors such as polycystic ovary syndrome (PCOS), endometriosis, and other reproductive health disorders. The increasing demand for minimally invasive gynecological procedures is further accelerating adoption, as women increasingly seek treatments that minimize pain, recovery time, and the need for hospitalization.
According to recent studies (2024), more than 18 million women aged 30-55 years report excessive menstrual bleeding, often accompanied by anemia and impaired quality of life. For these patients, endometrial ablation offers an effective and less invasive alternative to hysterectomy. Devices such as the updated NovaSure V5 system are designed to accommodate a wide range of cervical and uterine anatomies, expanding access to treatment and improving patient outcomes.
Beyond HMB, broader reproductive health challenges are shaping market demand. Globally, approximately 73 million induced abortions occur annually, representing 61% of unintended pregnancies. While abortion and ablation are distinct interventions, these figures underscore systemic gaps in menstrual and reproductive health management. For women who are not planning future pregnancies, ablation provides a permanent, non-hormonal solution that reduces the burden of heavy bleeding and helps improve reproductive health management strategies.
PCOS, which affects 8-13% of women of reproductive age, with nearly 70% remaining undiagnosed, remains a critical driver of demand. As PCOS is a leading cause of anovulation and irregular menstrual cycles, it often results in chronic endometrial stimulation and heavy bleeding. For patients not pursuing fertility, endometrial ablation can be an effective management tool, reducing complications and improving quality of life. Similarly, endometriosis, which affects nearly 10% of reproductive-age women worldwide (≈190 million), contributes to the need for advanced uterine treatment devices, as many patients suffer from debilitating menstrual pain and bleeding that may be alleviated through ablation procedures.
The market is also being shaped by technological innovation and regulatory milestones. For instance, in February 2023, Hologic’s NovaSure V5 endometrial ablation device received approval in Canada and Europe, featuring enhanced design elements that improve safety and efficacy while expanding the pool of eligible patients. Ongoing R&D investments by leading companies are expected to yield further device refinements, supporting market expansion.
Despite these opportunities, the market faces notable challenges. Endometrial ablation is not suitable for women seeking to preserve fertility, as it irreversibly damages the uterine lining, limiting its use to women who have completed childbearing. This constraint is particularly relevant as many women increasingly delay pregnancy into their 30s and 40s. Moreover, while minimally invasive, the procedure carries risks, including infection, uterine perforation, post-ablation pain, or injury to adjacent organs, which can discourage adoption among patients and providers. Coupled with variations in reimbursement policies and access to advanced gynecological care across regions, these limitations may temper future growth.
Overall, the global endometrial ablation devices market is poised for continued expansion, supported by a large and underdiagnosed patient population, growing awareness of minimally invasive alternatives, and ongoing product innovation. However, fertility limitations, safety concerns, and regional disparities in access will remain critical factors shaping adoption patterns and long-term market dynamics.
Endometrial Ablation Devices Market Segment Analysis
Endometrial Ablation Devices Market by Product Type (Radiofrequency Ablation Devices, Thermal Balloon Ablation Devices, Cryoablation Devices, Hydrothermal Ablation Devices, Microwave Ablation Devices, and Others), End-Users (Hospitals, Specialty Clinics, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World)
By Product Type: Radiofrequency Ablation Devices Category Dominates the Market
In the product type segment of the endometrial ablation devices market, radiofrequency ablation devices are projected to hold the largest market share of 55% in 2024, due to several key advantages that make them a preferred choice among healthcare providers and patients. Radiofrequency ablation offers precise and uniform destruction of the endometrial lining, leading to more consistent treatment outcomes and higher patient satisfaction compared to other methods. These devices generally allow for shorter procedure times, reduced patient discomfort, and faster recovery, aligning well with the growing demand for minimally invasive outpatient treatments. For example, the Minerva Endometrial Ablation System by Minerva Surgical utilizes advanced radiofrequency technology combined with saline infusion to ensure effective and controlled ablation. This device includes safety features such as real-time tissue impedance monitoring and temperature control to minimize risks like uterine perforation or excessive thermal damage. The widespread availability of such radiofrequency devices, along with strong clinical evidence supporting their safety and efficacy, further drives adoption. Leading companies like Minerva Surgical continue to invest heavily in research and development, enhancing device usability and expanding applicability to accommodate diverse patient anatomies. These factors collectively contribute to radiofrequency ablation devices maintaining dominance in the market, especially as healthcare systems worldwide prioritize cost-effective, efficient, and patient-friendly solutions for managing heavy menstrual bleeding and related conditions.
By End-User: Hospitals Dominate the Market
Hospitals play a crucial role in boosting the endometrial ablation devices market by serving as primary centers for diagnosis, treatment, and follow-up care of women with heavy menstrual bleeding and related conditions. Their well-equipped infrastructure and skilled healthcare professionals enable the safe and effective use of advanced ablation technologies. Additionally, hospitals often lead in adopting new medical devices due to their access to training and research resources, helping drive wider acceptance and trust among patients and providers. As more hospitals expand outpatient and minimally invasive treatment services, the demand for endometrial ablation devices increases, directly contributing to market growth.
Endometrial Ablation Devices Market Regional Analysis
North America Endometrial Ablation Devices Market Trends
North America is projected to capture the largest share of 38% in the global Endometrial Ablation Devices market in 2024, supported by the high prevalence of abnormal uterine bleeding (AUB), particularly among women aged 35-55, and the presence of favorable reimbursement frameworks. Strong awareness of women’s health issues, coupled with continuous product development and regulatory approvals, further reinforces the region’s leadership in this segment.
Heavy menstrual bleeding (HMB) remains a major driver of market demand in North America. According to the Centers for Disease Control and Prevention (2025), more than 10 million women in the U.S. experience HMB annually, roughly one in every five women of reproductive age. Beyond disrupting daily life, HMB can cause long-term complications such as iron-deficiency anemia, creating a large and consistent treatment-seeking population. Endometrial ablation devices, which offer a minimally invasive alternative to hysterectomy by thermally destroying the uterine lining, are increasingly being adopted as a first-line intervention, especially as patient and provider awareness rises.
The growing burden of polycystic ovary syndrome (PCOS) further expands the potential market. Recent studies (2025) estimate PCOS affects between 5% and 20% of reproductive-age women globally, with North American prevalence estimated at one in seven women. Since PCOS is frequently associated with irregular or heavy menstrual bleeding, it significantly contributes to the demand for effective, long-term treatment options. For women not planning future pregnancies, endometrial ablation provides a durable, non-hormonal solution that improves quality of life and reduces reliance on pharmaceutical management, thereby driving adoption among this underserved population.
Regulatory approvals and technological advancements are also key growth accelerators. For instance, in July 2023, the FDA approved the Minitouch 3.8 Era System by MicroCube, LLC, a device that uses thermal energy, either cold or heat, to ablate the entire uterine lining. Such innovations not only broaden the treatment toolkit available to clinicians but also enhance safety, efficacy, and usability across diverse patient populations. With a growing pipeline of devices incorporating improved safety features, ergonomic designs, and broader anatomical compatibility, healthcare providers have more flexibility to tailor therapies to individual patient needs, thereby strengthening adoption trends.
Taken together, the region’s large patient pool, favorable reimbursement environment, increasing awareness of women’s health, and continuous flow of innovative product approvals are expected to sustain North America’s dominant position in the global endometrial ablation devices market throughout the forecast period.
Europe Endometrial Ablation Devices Market Trends
Europe plays a significant role in driving the growth of the endometrial ablation devices market, largely due to the high prevalence of heavy menstrual bleeding (HMB) among women of reproductive age. According to recent studies in 2025, approximately one in three women in Europe reports experiencing HMB, a condition that severely impacts quality of life and daily functioning. This substantial patient base creates a strong demand for effective and minimally invasive treatment options, positioning endometrial ablation devices as a preferred choice across the region.
The European healthcare infrastructure is well-developed, with increasing adoption of advanced medical technologies and growing awareness among both healthcare providers and patients about less invasive alternatives to traditional surgical treatments like hysterectomy. Hospitals and outpatient clinics in Europe are expanding their use of endometrial ablation procedures due to shorter recovery times, reduced complications, and improved patient satisfaction. This trend is further supported by favorable reimbursement policies and regulatory frameworks that encourage the adoption of innovative medical devices.
Moreover, continuous research and clinical trials in Europe contribute to the development of improved endometrial ablation technologies tailored to diverse patient anatomies and needs. The combination of a large affected population, progressive healthcare systems, and ongoing innovation drives strong market growth in Europe. As more women seek effective solutions for managing HMB, the demand for endometrial ablation devices is expected to rise steadily, making Europe a critical region for market expansion and innovation in this field.
Asia-Pacific Endometrial Ablation Devices Market Trends
The Asia-Pacific region is rapidly emerging as a key growth driver in the endometrial ablation devices market, fueled by the high prevalence of heavy menstrual bleeding (HMB) and increasing healthcare awareness across countries like India and China. In India, HMB is one of the leading causes of gynecological visits, with prevalence rates reported between 17.9% and 36% in various studies. Similarly, research in China indicates that HMB affects between 18.2% and over 20% of women of reproductive age, highlighting a substantial patient population in need of effective treatment options.
This significant burden of HMB in the region creates a large and growing demand for minimally invasive therapies like endometrial ablation, which offer benefits such as reduced recovery time and fewer complications compared to traditional surgical interventions. As healthcare infrastructure improves and access to advanced medical technologies expands, more hospitals and clinics in the Asia-Pacific are adopting these devices to meet patient needs. Furthermore, rising awareness among women and healthcare providers about treatment alternatives beyond medication or hysterectomy is driving market acceptance.
In addition, favorable government initiatives and increasing healthcare expenditure in countries within the region support the introduction and reimbursement of innovative medical devices. Combined with ongoing product innovations tailored to diverse patient anatomies, these factors position the Asia-Pacific as a rapidly growing market for endometrial ablation devices. As more women seek effective management for HMB, the region is expected to play a pivotal role in the global expansion of this market.
Who are the major players in the endometrial ablation devices market?
The following are the leading companies in the endometrial ablation devices market. These companies collectively hold the largest market share and dictate industry trends.
- Medtronic
- Johnson & Johnson Services Inc.
- Olympus Corporation
- Minerva Surgical, Inc.
- Hologic, Inc.
- CooperSurgical Inc.
- Richard Wolf GmbH
- AngioDynamics
- KARL STORZ SE & Co. KG
- IDOMAN-MED
- Arthrex, Inc.
- Channel Medsystems, Inc.
- Gynesonics
- Boston Scientific Corporation
- AEGEA Medical
- Others
How is the competitive landscape shaping the Endometrial Ablation Devices market?
The competitive landscape of the endometrial ablation devices market is characterized by a mix of well-established global players and emerging companies, resulting in a moderately concentrated market. Leading manufacturers, such as Hologic, Minerva Surgical, and MicroCube, LLC, dominate the space by leveraging strong brand recognition, extensive clinical data, and continuous innovation to maintain their market positions. These key players invest heavily in research and development to enhance device safety, efficacy, and ease of use, which helps them capture significant market share and set high barriers to entry for new competitors. At the same time, emerging companies and startups are entering the market with novel technologies and cost-effective solutions, intensifying competition and driving innovation. This dynamic competitive environment encourages continuous product improvements and expanded indications, ultimately benefiting healthcare providers and patients. Overall, while the market remains dominated by a few key players, the presence of innovative challengers and increasing adoption worldwide contribute to healthy competition and steady market growth.
Recent Developmental Activities in the Endometrial Ablation Devices Market
- In May 2025, Boston Scientific Corporation announced U.S. Food and Drug Administration (FDA) approval of its Genesys HTA™ System for the treatment of menorrhagia. The Genesys HTA System is a next-generation endometrial ablation system designed to ablate the endometrial lining of the uterus in premenopausal women with menorrhagia.
- In October 2024, Hologic, Inc., a global champion of women’s health, announced that it had signed a definitive agreement to acquire Gynesonics, Inc. (Gynesonics®), a privately held medical device company focused on the development of minimally invasive solutions for women’s health.
- In July 2023, the Minitouch 3.8 Era System, manufactured by MicroCube, LLC, marked a significant advancement in the endometrial ablation device market by receiving FDA approval.
- In February 2023, Hologic's NovaSure V5 endometrial ablation device received approval for use in Canada and Europe.
|
Report Metrics |
Details |
|
Study Period |
2022 to 2032 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2032 |
|
Endometrial Ablation Devices Market CAGR |
5.21% |
|
Key Companies in the Endometrial Ablation Devices Market |
Medtronic, Johnson & Johnson Services, Inc., Olympus Corporation, Minerva Surgical, Inc., Hologic, Inc., CooperSurgical Inc., Richard Wolf GmbH, AngioDynamics, KARL STORZ SE & Co. KG, IDOMAN-MED, Arthrex, Inc., Channel Medsystems, Inc., Gynesonics, Boston Scientific Corporation, AEGEA Medical, and others. |
|
Endometrial Ablation Devices Market Segments |
by Product Type, by End-user, and by Geography |
|
Endometrial Ablation Devices Regional Scope |
North America, Europe, Asia Pacific, Middle East, Africa, and South America |
|
Endometrial Ablation Devices Country Scope |
U.S., Canada, Mexico, Germany, United Kingdom, France, Italy, Spain, China, Japan, India, Australia, South Korea, and key Countries |
Endometrial Ablation Devices Market Segmentation
- Endometrial Ablation Devices by Product Type Exposure
- Radiofrequency Ablation Devices
- Thermal Balloon Ablation Devices
- Cryoablation Devices
- Hydrothermal Ablation Devices
- Microwave Ablation Devices
- Others
- Endometrial Ablation Devices End-Users Exposure
- Hospitals
- Specialty Clinics
- Others
- Endometrial Ablation Devices Geography Exposure
- North America Endometrial Ablation Devices Market
- United States Endometrial Ablation Devices Market
- Canada Endometrial Ablation Devices Market
- Mexico Endometrial Ablation Devices Market
- Europe Endometrial Ablation Devices Market
- United Kingdom Endometrial Ablation Devices Market
- Germany Endometrial Ablation Devices Market
- France Endometrial Ablation Devices Market
- Italy Endometrial Ablation Devices Market
- Spain Endometrial Ablation Devices Market
- Rest of Europe Endometrial Ablation Devices Market
- Asia-Pacific Endometrial Ablation Devices Market
- China Endometrial Ablation Devices Market
- Japan Endometrial Ablation Devices Market
- India Endometrial Ablation Devices Market
- Australia Endometrial Ablation Devices Market
- South Korea Endometrial Ablation Devices Market
- Rest of Asia-Pacific Endometrial Ablation Devices Market
- Rest of the World Endometrial Ablation Devices Market
- South America Endometrial Ablation Devices Market
- Middle East Endometrial Ablation Devices Market
- Africa Endometrial Ablation Devices Market
- North America Endometrial Ablation Devices Market
Endometrial Ablation Devices Market Recent Industry Trends and Milestones (2022-2025)
|
Category |
Key Developments |
|
Endometrial Ablation Devices Regulatory Approvals |
Boston Scientific Corporation - Genesys HTA™ System (FDA), MicroCube, LLC - Minitouch 3.8 Era System (FDA), Hologic's NovaSure V5 endometrial ablation device received approval for use in Canada and Europe. |
|
Partnerships in the Endometrial Ablation Devices Market |
Hologic, Inc. announced a strategic partnership with Olympus Corporation to co-develop integrated hysteroscopic visualization and endometrial ablation solutions. Minerva Surgical partnered with Blackmaple Group to offer minimally invasive gynecologic technologies to WHAAPA physician members. The agreement includes the Minerva ES Ablation System and Medical Endoscopy Image Processing System for detecting and treating abnormal uterine bleeding (AUB). |
|
Acquisition in the Endometrial Ablation Devices Market |
CooperSurgical acquired AEGEA Medical, which included its Mara Water Vapor Ablation System. This was a major move to add a water-vapor‑based ablation modality to its women’s health portfolio. |
|
Company Strategy |
Minerva Surgical, Inc.: Broadening the portfolio to offer not just ablation but visualization, tissue removal, and diagnostics in-office and in-hospital settings. Hologic, Inc.: Securing patents for improved radiofrequency ablation systems that modulate power to match tissue impedance. Medtronic: Developing and offering devices that can tap into multiple types of ablation technologies to address different uterine anatomies and patient needs. |
|
Emerging Technology |
Cryotherapy / Cryoablation Devices, Microwave Ablation, Integration of Real‑Time Imaging / Navigation / Sensing, Minimally Invasive & Office‑Based Devices |
Impact Analysis
AI-Powered Innovations and Applications:
AI is increasingly being integrated into gynecologic imaging, diagnostics, treatment planning, and device feedback loops, and these advances are poised to benefit endometrial ablation devices in multiple ways. For example, deep learning models are now able to detect and classify endometrial polyps in real time from hysteroscopic video frames with very high accuracy (e.g., sensitivity ~96%, with strong precision and mean average precision metrics), which could help in ensuring that ablation is applied only to intended tissue and reduce over‑treatment or missed pathology. Also, convolutional neural networks trained on hysteroscopic images have been used to predict fertility outcomes (such as after treatment of intrauterine adhesions / endometrial injury), allowing quantifiable visualization panels of intrauterine pathology to guide pre‑procedure patient selection and post‑procedure follow‑up. AI is also used in related fields to better segment uterine myomas preoperatively using MRI, shortening operation times and reducing intraoperative blood loss, which suggests that, similarly, AI could help map uterine anatomy more precisely before ablation, improving device placement and treatment efficiency.
On the technical side, there is work like PhysRFANet, a physics‑guided neural network model that can predict the thermal effects of radiofrequency ablation in real time (temperature distribution and lesion volume) with high accuracy and fast inference. While this is demonstrated in contexts like tumor ablation, such models could be adapted to endometrial ablation to help ensure that thermal ablation is effective but safe, avoiding under‑ or over‑treatment. Additionally, device manufacturers (for example, Hologic) are patenting systems that modulate radiofrequency power based on tissue impedance, which can be seen as a kind of feedback loop that an AI/ML system could enhance, to dynamically adjust energy delivery for safety and efficacy.
U.S. Tariff Impact Analysis on Endometrial Ablation Devices Market:
U.S. tariffs are creating notable challenges for the endometrial ablation devices market by increasing the cost of importing key components and finished products, particularly from countries like China, the European Union, and others affected by trade policies. Since many of these devices rely on globally sourced materials—such as specialized electronics, sensors, and plastics tariffs add a financial burden to manufacturers, forcing them to either absorb the extra cost or pass it on to hospitals, clinics, and ultimately, patients. This can result in higher device prices, making advanced ablation technologies less accessible, especially for smaller healthcare providers with limited budgets. In turn, this may slow down the adoption of newer and more innovative devices, particularly in outpatient and ambulatory settings where cost sensitivity is high. Furthermore, the unpredictability of tariff regulations makes long-term planning difficult for companies, discouraging investment in product development and innovation. While U.S.-based manufacturers could see some benefit from reduced competition and lower import-related costs, relocating manufacturing or restructuring supply chains to avoid tariffs is both expensive and time-consuming, often requiring new regulatory approvals. Over time, companies may be pushed to localize production or shift sourcing to tariff-exempt regions, but until then, the market may experience slower growth and reduced competitiveness. These tariff impacts could also prompt industry advocacy for medical device exemptions, as rising costs begin to affect both provider choices and patient access to essential gynecological treatments like endometrial ablation.
How This Analysis Helps Clients
- Cost Management: By understanding the tariff landscape, clients can anticipate cost increases and adjust pricing strategies accordingly, ensuring profitability.
- Supply Chain Optimization: Clients can identify alternative sourcing options and diversify their supply chains to reduce dependency on high-tariff regions, enhancing resilience.
- Regulatory Navigation: Expert guidance on navigating the evolving regulatory environment helps clients maintain compliance and avoid potential legal challenges.
- Strategic Planning: Insights into tariff impacts enable clients to make informed decisions about manufacturing locations, partnerships, and market entry strategies.
Key takeaways from the Endometrial Ablation Devices market report study
- Market size analysis for the current endometrial ablation devices market size (2024), and market forecast for 8 years (2025 to 2032)
- Top key product/technology developments, mergers, acquisitions, partnerships, and joint ventures happened over the last 3 years.
- Key companies dominating the endometrial ablation devices market.
- Various opportunities available for the other competitors in the endometrial ablation devices market space.
- What are the top-performing segments in 2024? How these segments will perform in 2032?
- Which are the top-performing regions and countries in the current endometrial ablation devices market scenario?
- Which are the regions and countries where companies should have concentrated on opportunities for the endometrial ablation devices market growth in the future?