Epidermal Growth Factor Receptor-Non Small Cell Lung Cancer (EGFR-NSCLC) Market
Key Highlights
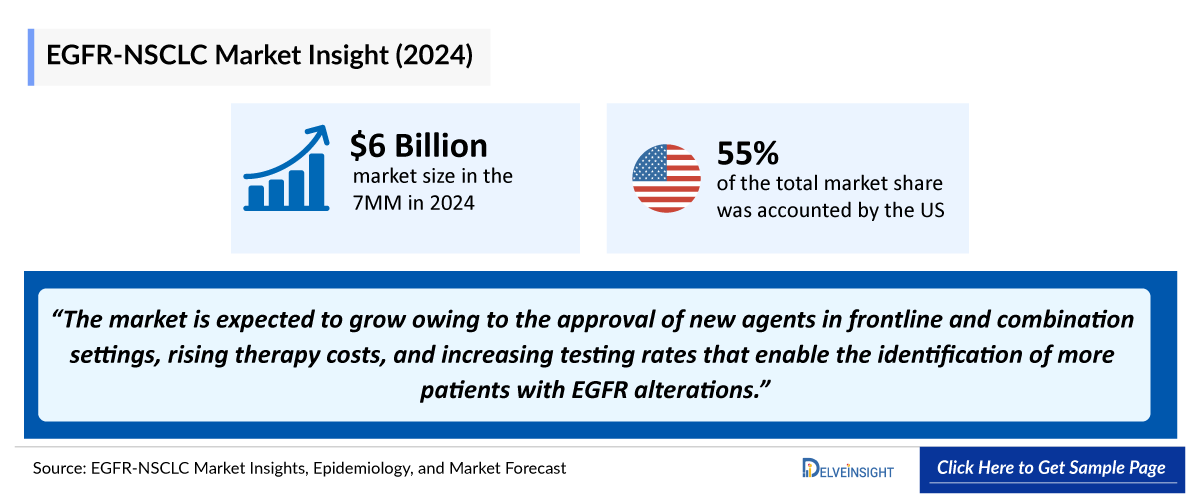
- The total market size of EGFR-NSCLC in the 7MM was ~USD 6,000 in 2024 and expected to increase in coming years owing to the approval of new agents in frontline and combination settings, rising therapy costs, and increasing testing rates that enable the identification of more patients with EGFR alterations
- Within EGFR-NSCLC, where osimertinib maintains market leadership, J&J’s amivantamab is steadily gaining ground. It has entered the broader first-line EGFR-mutated market through combination with the oral EGFR-Tyrosine Kinase Inhibitor lazertinib, and the company has launched a subcutaneous version of amivantamab + lazertinib in Europe, with a US launch expected soon. Given its expanding role and dual mechanism as an EGFR x cMET bispecific, amivantamab is well-positioned to evolve into a potential billion-dollar product.
- The market for NSCLC is increasingly driven by biomarkers, with EGFR being one of the most lucrative segments. Osimertinib, a leading therapy in the EGFR-NSCLC market, holds a dominant position. However, resistance to EGFR tyrosine kinase inhibitors is on the rise among patients. Addressing the needs of patients who develop resistance after Osimertinib treatment represents one of the most significant areas of unmet need.
- The ADC space in EGFR-NSCLC awaits more launches down the road, with several compounds under development in pivotal studies. The ADC space in EGFR-NSCLC awaits more launches down the road, with several compounds under development in pivotal studies. After the approval of Dato-Dxd, leading pharma players drive ADC innovation with agents such as izalontamab brengitecan (BMS), telisotuzumab adizutecan (AbbVie), and sacituzumab tirumotecan (Merck).
- The emerging pipeline of EGFR-NSCLC includes BG-60366, a first-in-class Chimeric Degradation Activation Compound (CDAC), representing a novel therapeutic class in EGFR-NSCLC beyond current bispecific antibodies and ADCs.
- The majority of the emerging key players focus on the Exon 20 insertion segment. EXKIVITY's withdrawal from this segment stands behind RYBREVANT in the current market. Companies like EQRx International/Hansoh Pharmaceutical, Merus/Betta Pharmaceuticals, and Taiho Oncology are aiming at the Exon-20 mutant EGFR-NSCLC segment.
DelveInsight’s “Epidermal Growth Factor Receptor Non-small Cell Lung Cancer (EGFR-NSCLC) – Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of EGFR-NSCLC, historical and forecasted epidemiology as well as EGFR-NSCLC market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The EGFR-NSCLC market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted the 7MM EGFR-NSCLC market size from 2020 to 2034. The report also covers current EGFR-NSCLC treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
EGFR-NSCLC Epidemiology
|
Segmented by:
(EGFR Exon 20 Insertion Mutations, EGFR exon 19 deletions [sensitizing mutations], Exon 21 L858R substitution [sensitizing mutations], Other submutation [EGFR exon 20 p.S768I, exon 21 p.L861Q, and exon 18 p.G719X Alterations, etc.])
|
|
EGFR-NSCLC Key companies |
AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hansoh Pharmaceutical, Johnson & Johnson Innovative Medicine, Pfizer, Cullinan Oncology, Taiho Pharmaceutical, ArriVent Biopharma, Black Diamond Therapeutics, Daiichi Sankyo, Akeso Biopharma, Summit Therapeutics, and others |
|
EGFR-NSCLC Key therapies |
Osimertinib (TAGRISSO), Amivantamab (RYBREVANT), Sunvozertinib (ZEGFROVY), Aumolertinib (AUMSEQA), Dato-Dxd (DATROWAY), Zipalertinib, Ivonescimab, Pamvatamig (MCLA-129), Furmonertinib, BDTX-1535, and others |
|
EGFR-NSCLC Market |
Segmented by:
|
|
Analysis |
|
Epidermal Growth Factor Receptor Non-small Cell Lung Cancer Disease Understanding and Treatment Algorithm
EGFR Metastatic Non-small Cell Lung Cancer Overview
EGFR is a protein in cells that helps them grow. A mutation in the gene for EGFR can make it grow too much, which can cause cancer. There are different types of EGFR mutations, including deletions or insertions and point mutations. In test results, individuals may be identified as having an EGFR 19 deletion or an EGFR L858R point mutation, which are the most common types of EGFR mutations. These mutations are typically treated the same way. Amongst the EGFR mutations that are tested for in lung cancer, a few rare types are treated differently than the more common EGFR mutations. The major example of this in lung cancer is EGFR exon 20 insertions. This is a type of EGFR mutation that does not respond to the typical treatment for EGFR-positive lung cancer, which are called tyrosine kinase inhibitors, or TKIs.
EGFR Non-small Cell Lung Cancer Diagnosis
In general, there are two ways to detect EGFR mutations. The best way is through comprehensive next-generation sequencing (NGS). This type of testing places tissue from a patient’s tumor (gathered from a biopsy) in a machine that looks for a large number of possible biomarkers at one time. There may be some situations where a patient cannot undergo the biopsy needed to perform NGS, so liquid biopsy is recommended. A liquid biopsy can look for certain biomarkers in a patient’s blood.
Further details related to diagnosis are provided in the report…
EGFR Non-small Cell Lung Cancer Treatment
Treatment options and recommendations depend on several factors, including the type and stage of cancer, possible side effects, and the patient’s preferences and overall health. The most common treatments for EGFR non-small cell lung cancer are:
|
Molecular and Biomarker-directed Therapy for EGFR-NSCLC | |
|
EGFR Exon 19 Deletion or Exon 21 L858R | |
|
First-line therapy |
Afatinib |
|
Erlotinib | |
|
Dacomitinib | |
|
Gefitinib | |
|
Osimertinib | |
|
Subsequent therapy |
Osimertinib |
|
EGFR S768I, L861Q, and/or G719X | |
|
First-line therapy |
Afatinib |
|
Erlotinib | |
|
Dacomitinib | |
|
Gefitinib | |
|
Osimertinib | |
|
Subsequent therapy |
Osimertinib |
|
EGFR exon 20 insertion mutation | |
|
Subsequent therapy |
Amivantamab-vmjw |
|
Sunvozertinib | |
Epidermal Growth Factor Receptor Non-small Cell Lung Cancer Epidemiology
The EGFR Non-small Cell Lung Cancer epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Incident Cases of NSCLC, Gender-specific Cases of NSCLC, Age-specific Cases of NSCLC, Total Incident Cases of NSCLC by Histology, Total Incident Cases of NSCLC by Stage, Total Cases of EGFR-positive NSCLC, EGFR-positive NSCLC incident cases by Subtypes, and Line-wise Treated Cases of EGFR-positive NSCLC in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
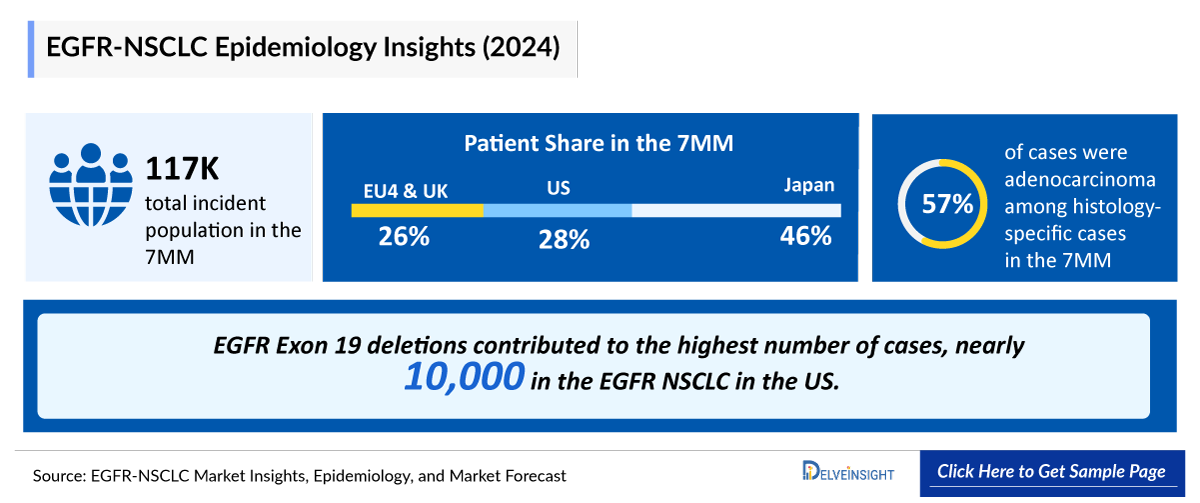
- The total number of incident cases of NSCLC in the United States was nearly 204,800 in 2024.
- The total number of cases in EU4 and the UK for EGFR-NSCLC was estimated to be nearly 211,000 cases in 2024.
- Among the total NSCLC cases, EGFR mutations represented the most prevalent biomarker, accounting for 50% of cases, followed by EGFR exon 19 deletions (sensitizing/classical mutations) at 15%, while PACC mutations (G719X, S768I, L792X, T854I, etc.) were the least common, comprising 5%.
- The total number of cases of EGFR Exon 20 insertion mutations in Japan was estimated to be nearly 5,000 in 2024.
Epidermal Growth Factor Receptor Non-small Cell Lung Cancer Drug Chapters
The drug chapter segment of the EGFR Non-small Cell Lung Cancer report encloses a detailed analysis of the marketed and the late-stage (Phase III) pipeline drug. The marketed drugs segment encloses drugs such as Osimertinib (TAGRISSO), Amivantamab (RYBREVANT), Sunvozertinib (ZEGFROVY), Aumolertinib (AUMSEQA), and others. Furthermore, the current key players for the upcoming emerging drugs and their respective drug candidates include Taiho Pharmaceutical/Cullinan Oncology (zipalertinib), Arrivent Biopharma (furmonertinib), and others. The drug chapter also helps understand the EGFR-NSCLC clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
Marketed Drugs
Osimertinib (TAGRISSO): AstraZeneca
Osimertinib is a prescription medicine for adults with NSCLC with abnormal EGFR genes. It is used to prevent recurrence after surgery, as a first-line treatment for metastatic NSCLC, or when previous EGFR TKI treatments have failed. Osimertinib is a kinase inhibitor that targets mutant EGFR forms (T790M, L858R, exon 19 deletions) at lower concentrations than wild-type EGFR. In November 2015, it was initially approved 80mg once-daily tablets for the treatment of patients with metastatic EGFR T790M mutation-positive NSCLC. In February 2024, the FDA approved Osimertinib with platinum-based chemotherapy for patients with locally advanced or metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations.
In September 2025, Osimertinib plus chemotherapy demonstrated a median overall survival of nearly 4 years, the longest benefit ever reported in a global Phase III trial in EGFR-mutated advanced lung cancer.
In July 2025, Osimertinib plus chemotherapy demonstrated statistically significant and clinically meaningful improvement in overall survival in EGFR-mutated advanced lung cancer.
Sunvozertinib (ZEGFROVY): Dizal Pharmaceutical
Sunvozertinib is an oral, irreversible kinase inhibitor specifically approved for the treatment of adults with locally advanced or NSCLC harboring EGFR exon 20 insertion mutations, as confirmed by an FDA-approved test, whose disease has progressed after platinum-based chemotherapy. By selectively inhibiting mutant EGFR and showing reduced activity on wild-type EGFR, sunvozertinib effectively disrupts oncogenic signaling pathways that drive tumor growth, while generally limiting toxicity commonly associated with EGFR-targeted therapies.
In July 2025, the US FDA granted an accelerated approval to sunvozertinib as the only targeted oral therapy for NSCLC with EGFR Exon 20 insertion mutations.
|
Comparison of Marketed Drugs | |||||
|
Product |
Company |
Combination |
RoA |
MoA |
Approval |
|
Osimertinib (TAGRISSO) |
AstraZeneca |
Monotherapy |
Oral |
EGFR TKI |
US: 2015 (for second-line and above treatment of EGFR T790M mutation-positive metastatic non-small cell lung cancer); 2018 (for first-line treatment of EGFR non-small cell lung cancer) EU4 and the UK: 2016 (for second-line and above treatment of EGFR T790M mutation-positive metastatic non-small cell lung cancer); 2018 (for first-line treatment of EGFR non-small cell lung cancer); 2024 (addition of pemetrexed and platinum-based chemotherapy for first-line treatment of EGFR-NSCLC). JP: 2016 (for the second-line and above treatment of EGFR T790M mutation-positive metastatic non-small cell lung cancer); 2018 (for first-line treatment of EGFR non-small cell lung cancer); 2024 (addition of pemetrexed and platinum-based chemotherapy for first-line treatment of EGFR-NSCLC). |
|
Amivantamab (RYBREVANT) |
Johnson & Johnson Innovative Medicine |
Monotherapy |
IV |
EGFR and MET inhibitor |
US: 2021 (for second-line and above treatment of EGFR non-small cell lung cancer); 2024 (for first-line treatment of advanced NSCLC with EGFR exon 20 insertions) EU4 and the UK: 2021 (for second-line and above treatment of EGFR non-small cell lung cancer) |
|
Sunvozertinib (ZEGFROVY) |
Dizal Pharmaceutical |
Monotherapy |
Oral |
Irreversible EGFR inhibitor |
US: 2025 (for first-line therapy for non-small cell lung cancer with EGFR exon 20 insertion mutations) |
Emerging Drugs
Zipalertinib: Cullinan Oncology/Taiho Pharmaceutical
Zipalertinib (CLN-081/TAS6417) is a novel, orally bioavailable, irreversible EGFR inhibitor that, based on preclinical models, selectively and potently targets cells expressing EGFRex20ins mutations while relatively sparing cells expressing wild-type EGFR to avoid the toxicities associated with inhibition of wild-type EGFR. This was rationally designed with a distinct chemical scaffold to be highly selective for mutant vs. wild-type EGFR and to avoid inhibiting the closely related receptor human epidermal growth factor receptor 2 (HER2). Zipalertinib demonstrates the potential to become a new standard of care to treat non-small cell lung cancer harboring EGFRex20ins mutations.
In September 2025, according to the company’s corporate presentation, Pending FDA discussions, a potential NDA filing by Taiho is targeted for YE 2025 in relapsed EGFR ex20ins NSCLC and randomized REZILIENT3 Phase III frontline trial is ongoing, with completion of enrolment expected in H1 2026.
Firmonertinib: ArriVent BioPharma
Firmonertinib (formerly furmonertinib), is an oral, highly brain-penetrant, and broadly active mutation-selective EGFR inhibitor active against both classical and uncommon EGFR mutations, including PACC and exon 20 insertion mutations.
Firmonertinib is currently being studied in a global Phase III trial for first-line NSCLC patients with EGFR exon 20 insertion mutations (FURVENT; NCT05607550) and in a global Phase Ib study evaluating firmonertinib in patients with EGFR PACC mutations (FURTHER; NCT05364043). In addition, firmonertinib is also being studied in a clinical combination study targeting advanced or metastatic NSCLC patients with EGFR classical mutations in partnership with InnoCare Pharma.
According to ArriVent BioPharma’s Second Quarter 2025 Financial Results, Top-line firmonertinib monotherapy data from the global pivotal FURVENT Phase III study for first-line EGFR exon20 insertion mutant NSCLC are projected to be in early 2026.
|
Comparison of Emerging Drugs Under Development for EGFR Non-small Cell Lung Cancer | ||||||
|
Product |
Company |
Mechanism of Action |
Phase |
Indication |
RoA |
Molecular Type |
|
Zipalertinib |
Taiho Pharmaceutical/ Cullinan Oncology |
Irreversible EGFR inhibitor |
III |
EGFR Exon 20 insertion non-small cell lung cancer |
Oral |
Small molecule |
|
Furmonertinib |
ArriVent Biopharma |
Mutation-selective EGFR inhibitor |
III |
EGFR mutations and HER2 exon 20 insertion non-small cell lung cancer |
Oral |
Small molecule |
|
Ivonescimab (SMT112) |
Akeso Biopharma/ Summit Therapeutics |
PD-1/VEGF inhibitor |
III |
Advanced NSCLC/EGFR mutant advanced NSCLC |
IV infusion |
Bispecific antibody |
|
Telisotuzumab Adizutecan (Temab-A/ ABB-400) |
AbbVie |
Anti-c-MET |
III/II |
EGFR ̶ NSCLC |
IV infusion |
ADC |
|
LP-300 |
Lantern Pharma |
EGFR inhibitor |
II |
EGFR ̶ NSCLC |
IV infusion |
Small molecule |
|
BG-60366 |
BeOne Medicines (formerly BeiGene) |
EGFR degrader |
I |
EGFR-mutant NSCLC |
Oral |
Small molecule |
Drug Class Insights
The existing EGFR non-small cell lung cancer is mainly dominated by targeted therapies for mutations such as EGFR-sensitizing mutations and EGFR exon 20 insertions. EGFR mutations are frequently observed, EGFR exon 19 deletions and EGFR exon 21 L858R mutations. The FDA has approved various tyrosine kinase inhibitors (TKIs) to treat these mutations.
First and second-generation EGFR TKI: Compared to platinum-based chemotherapy (i.e., cisplatin or carboplatin combined with either gemcitabine, pemetrexed, paclitaxel, or docetaxel), first- and second-generation EGFR TKIs have higher response rates (RRs) and progression-free survival (PFS).
Third-generation EGFR TKI: Osimertinib is the only targeted therapy that has shown survival benefits in both early- and late-stages of EGFR-NSCLC. Due to resistance development, osimertinib is recommended for patients with EGFR T790M-mutant NSCLC who progress after a first- or second-generation TKI.
TROP-2 directed ADC
TROP2-directed antibody-drug conjugates (ADCs) are a novel class of targeted cancer therapeutics that harness an anti-TROP2 monoclonal antibody to deliver highly potent cytotoxic payloads selectively to tumor cells expressing the TROP2 surface antigen. It is an emerging therapeutic class in EGFR-mutated NSCLC, particularly following resistance to EGFR tyrosine kinase inhibitors (TKIs). Dato-dxd is the first TROP2-ADC approved for adults with EGFR-mutant, locally advanced or metastatic NSCLC after progression on EGFR-targeted therapy and chemotherapy.
American Society of Clinical Oncology (ASCO) 2025
|
Drug name |
Company |
Highlights |
|
Patritumab Deruxtecan |
Daiichi Sankyo/ AstraZeneca |
Daiichi Sankyo presented Phase III HERTHENA-Lung02 results, where Patritumab Deruxtecan (HER3-DXd) improved PFS versus chemotherapy in EGFR-mutated NSCLC post-TKI, but did not meet the primary OS endpoint. |
|
Telisotuzumab Adizutecan (Temab-A/ABB-400) |
AbbVie |
AbbVie highlighted its oncology pipeline with oral presentations on novel ADCs, including Telisotuzumab Adizutecan (ABBV-400, Temab-A) in NSCLC, which showed an ORR of 63% and mDOR of ≥6 months in a Phase I trial. |
|
Zipalertinib (CLN-081) |
Cullinan Oncology/ Taiho Pharma |
Taiho Oncology and Cullinan Therapeutics reported Phase IIb REZILIENT1 trial results showing zipalertinib monotherapy achieved a cORR of 35.2% and mDOR of 8.8 months in previously treated NSCLC patients with EGFR exon 20 insertion mutations. |
Note: Detailed insights will be provided in the final report.
Epidermal Growth Factor Receptor Non-small Cell Lung Cancer Market Outlook
The treatment of EGFR-mutant non-small cell lung cancer has been transformed by the development of targeted therapies in the last two decades; however, choosing the best therapy after EGFR TKIs fail is still a challenge. There are non-small cell lung cancers with common EGFR-sensitizing mutations (e.g., EGFR exon 19 deletions or exon 21 mutations [L858R]): afatinib and osimertinib. These drugs have different efficacy and safety profiles and are classified as first- (e.g., erlotinib, gefitinib), second- (e.g., afatinib, dacomitinib), or third-generation (e.g., osimertinib) TKIs. Afatinib and osimertinib, which are second- and third-generation TKIs, respectively, have shown prolonged activity against some rare EGFR mutations (e.g., T790M [osimertinib], G719X, L861Q, or S768I [afatinib and osimertinib]).
Gefitinib was the first EGFR TKI, gaining approval in Japan in 2002 for advanced NSCLC, followed by erlotinib in the US in 2004 and gefitinib in Europe in 2009. These first- and second-generation TKIs showed superior response rates and progression-free survival compared with platinum-based chemotherapy, though resistance limited durability. Afatinib, the first second-generation TKI approved by the FDA in 2013, along with dacomitinib, demonstrated incremental efficacy, while osimertinib set a new standard with superior PFS and OS outcomes, particularly in patients with T790M mutations, as confirmed in the AURA3 trial.
Despite these advances, no prospective head-to-head trials have directly compared second- and third-generation EGFR TKIs. While later-generation agents clearly outperform earlier ones in efficacy and safety, acquired resistance remains a major limitation. This underscores the evolving therapeutic landscape, where successive generations of EGFR TKIs have significantly improved outcomes in advanced NSCLC, but overcoming resistance remains the key challenge in optimizing long-term benefit.
Many companies, such as ArriVent Biopharma (furmonertinib), and Cullinan Oncology/Taiho Pharmaceuticals (zipalertinib) are developing third-generation EGFR TKIs for exon 20 insertion in non-small cell lung cancer. These drugs will compete with each other for this niche market.
- The total market size in the US for EGFR-NSCLC was estimated to be nearly USD 3,300 million in 2024, which is expected to increase due to the launch of emerging therapies and label expansion of current therapies.
- The EGFRm NSCLC space in 2025 is more competitive than ever, with the arrival of three new therapies Dato-DXd, sunvozertinib, and aumolertinib that expand treatment options and challenge established players like osimertinib and amivantamab.
Epidermal Growth Factor Receptor Metastatic Non-small Cell Lung Cancer Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, efficacy data, and order of entry.
It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake. By overcoming the resistance from first and second-generation EGFR inhibitors, and better efficacy in terms of overall response and progression-free survival, osimertinib became the market leader in the EGFR-NSCLC market.
Further detailed analysis of emerging therapies drug uptake in the report…
Epidermal Growth Factor Receptor Non-small Cell Lung Cancer Activities
The report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Non-small Cell Lung Cancer emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Medical Oncologists, Pulmonologists and Professors, Chief of the Thoracic Service at the Memorial Sloan Kettering Cancer Center, and Others.
Delveinsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as University of Southern California, Santa Maria della Misericordia Hospital, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Non-small Cell Lung Cancer market trends.
|
KOL Views |
|
“Patients with EGFR exon 20 insertion mutations need more effective therapies. Although second-line agents are approved for this population, they are associated with toxicities, and the mechanism of disease progression on these agents is unclear.” ̶ Professor, University of Southern California, US |
|
“Osimertinib demonstrates superior CNS activity compared with first-generation TKIs; however, its median PFS in patients with brain metastases is only around 13 months, and leptomeningeal disease remains a significant site of progression.” ̶ PhD King's College London, UK |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most crucial primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and this clearly explains the drug's side effects in the trials. In addition, the scoring is also based on the probability of success and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
The cost of treating EGFR Non-small Cell Lung Cancer has shown significant increases over time, irrespective of the stage of the disease. This is particularly true for younger patients treated in the outpatient setting, according to real-world findings. The first-generation epidermal growth factor receptor tyrosine kinase inhibitors were reimbursed and available in all countries.
The TAGRISSO Patient Savings Program aims to assist eligible, commercially insured patients with their out-of-pocket costs for Osimertinib. Most eligible patients will pay USD 0 per month and may have access to up to USD 26,000 per year to assist with Osimertinib out-of-pocket costs. There are no income requirements to participate in the program.
Scope of the Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of EGFR-NSCLC, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the EGFR-NSCLC market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM EGFR Non-small Cell Lung Cancer market.
Epidermal Growth Factor Receptor Metastatic Non-small Cell Lung Cancer Report Insights
- Patient Population
- Therapeutic Approaches
- EGFR-NSCLC Pipeline Analysis
- EGFR-NSCLC Market Size and Trends
- Existing and Future Market Opportunity
Report Key Strengths
- Eleven Years Forecast
- The 7MM Coverage
- Non-small Cell Lung Cancer Epidemiology Segmentation
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
Epidermal Growth Factor Receptor Metastatic Non-small Cell Lung Cancer Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and conjoint Analysis)
FAQs
- What is the historical and forecasted EGFR-NSCLC patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What was the EGFR-NSCLC total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- Which class is going to be the largest contributor by 2034?
- What will be the impact of Osimertinib’s expected patent expiry?
- How will Osimertinib compete with Amivantamab in the first- and second lines?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- How many key players are developing therapies for exon 20 insertion EGFR-NSCLC?
- Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the EGFR-NSCLC Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.