Fungal Pneumonia Market
- The Fungal Pneumonia market is anticipated to experience growth during the forecast period (2023–2032). Growth of the global Fungal Pneumonia market is because fungal infections are more common in elderly people, pregnant women, burn victims, and those with diabetes. As the global geriatric population grows, the demand for fungal infection treatments is expected to rise. Fungal infections can be treated either systemically or topically. Over the next decade, increasing product launches will open up new doors of opportunity for the global fungal infections market. Decreased immune responses of an increasing number of people affected by cancer chemotherapy agents or acquired immunodeficiency syndrome have also contributed to the revenue growth of the fungal infection market.
- To drive the Fungal Pneumonia market in the future years, companies like Pfizer, Merck & Co., Gilead Sciences, Astellas Pharma, F2G Ltd, Cidara Therapeutics, Basilea Pharmaceutica, Scynexis, Inc., Matinas BioPharma, Viamet Pharmaceuticals, and others are developing their assets. With the expected approval of all these therapies currently under development, the overall therapeutic market of Fungal Pneumonia is likely to rise at a significant CAGR during the forecast period (2023–2032).
Request for Sample Page @ Fungal Pneumonia Market Report
DelveInsight’s report titled “Fungal Pneumonia Market Insights, Epidemiology, and Market Forecast – 2032” comprehensively analyzes historical and projected epidemiological data concerning Fungal Pneumonia. This analysis includes a breakdown of the Incident Cases of Fungal Pneumonia, Fungus-specific Incident Cases of Fungal Pneumonia, and Treatable Cases of Fungal Pneumonia. The Fungal Pneumonia market report offers an in-depth understanding of the various aspects related to the patient population, including diagnosis, prescription patterns, physician perspectives, market access, treatment, and future market developments for the seven major markets, including the United States, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan from 2019 to 2032.
The report examines the current treatment practices and algorithms for Fungal Pneumonia to assess the market’s potential and uncover potential business opportunities. The report also addresses the unmet medical needs in the field, aiming to identify areas where there is room for improvement and opportunities for innovation.
|
Study Period |
2019–2032 |
|
Forecast Period |
2023–2032 |
|
Geographies Covered |
The US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Fungal Pneumonia epidemiology |
|
|
Fungal Pneumonia Market |
|
|
Market Analysis |
|
|
Fungal Pneumonia Market Players |
Pfizer, Merck & Co., Gilead Sciences, Astellas Pharma, F2G Ltd, Cidara Therapeutics, Basilea Pharmaceutica, Scynexis, Inc., Matinas BioPharma, Viamet Pharmaceuticals, and others |
|
Treatment Class |
|
Fungal Pneumonia Treatment Market
Fungal Pneumonia Overview
Fungal Pneumonia is a non-contagious lung infection caused by fungal spores. It happens when the spores mix with the air and are inhaled or when an inactive infection is reactivated. Fungal pneumonia symptoms are those of the flu: coughing, headache, thick mucus, fever, and chest pain.
Several fungi cause fungal Pneumonia, the three most common ones being Pneumocystis, Cryptococcus, and Aspergillus. These fungi are found in the air, soil, and clinical environments like hospitals.
The fungi that cause fungal Pneumonia can be endemic (a fungus that’s native and restricted to a specific place) or opportunistic (a fungus that would not normally cause infections in otherwise healthy people but can cause an infection due to many factors, including organ transplant, cancer, immunodeficiency, etc.).
Fungal Pneumonia is not as common as other types of Pneumonia (like bacterial or viral), but it can be incredibly serious—even fatal—particularly in immunocompromised people. Fungal Pneumonia typically occurs in people with an abnormally low number of a specific type of white blood cell called neutropenia, but it also appears in patients with COPD, people with a history of long-term steroid use, and other demographics.
The symptoms of fungal Pneumonia are typically difficult to distinguish from other common respiratory ailments. People with fungal Pneumonia sometimes present with:
- Fever
- Cough (typically nonproductive)
- Chest pain or dull discomfort
- Rheumatologic (related to the joints) symptoms
- Hypersensitivity or allergic reactions
In neutropenic or immunocompromised persons, a persistent fever may be an early warning sign of an infection.
Diagnosing fungal Pneumonia can be difficult, but the most effective methods of testing for fungal Pneumonia include:
- Microscopic examination
- Fungal culture (collecting a sample of respiratory fluids and testing them)
- Antigen (a toxin or other foreign substance that induces an immune response in the body) and antibody testing
- Molecular testing to detect the genetic material of the fungus causing the infection
Sometimes, x-rays and chest CT scans will be ordered to assist in detecting fungal masses that can develop in the lungs.
Fungal Pneumonia is primarily treated with antifungal medications. Specific dosages and administration methods vary based on the individual case and type of infection. Itraconazole is the most common medication indicated for blastomycosis, histoplasmosis, and aspergillosis infections. Fluconazole, a triazole with the trade name Diflucan, is not only used to treat cryptococcal infection and valley fever, but it is also indicated to prevent fungal Pneumonia in HIV/AIDS or organ transplant patients.
Amphotericin B injection: Of another class of antifungals called polyenes, these drugs with the brand names Abelcet and Ambisome are taken intravenously daily in severe cases of fungal Pneumonia. Trimethoprim/sulfamethoxazole, the combination of these antibiotics, sold under the names Bactrim, Septra, and Cotrim, is often indicated in cases of pneumocystis pneumonia. In severe cases, oxygen therapy to restore oxygen levels and breathing exercises to loosen mucus and strengthen the lungs might be ordered. In general, the duration of treatment for fungal Pneumonia can last up to a year.
In March 2023, Cidara Therapeutics announced that the US FDA approved REZZAYO (rezafungin for injection) for treating candidemia and invasive candidiasis in adults with limited or no alternative treatment options. In over a decade, REZZAYO is the first new treatment option approved for candidemia and invasive candidiasis patients.
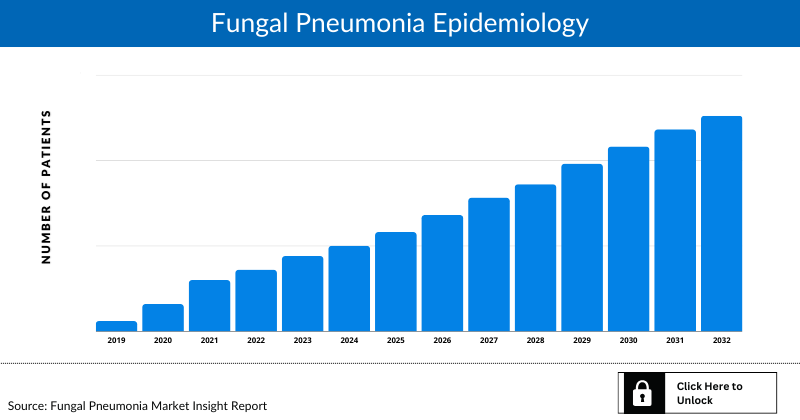
Fungal Pneumonia Epidemiology
The Fungal Pneumonia epidemiology section provides insights into the historical and current Fungal Pneumonia patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted trends by exploring numerous studies and key opinion leaders’ views. This part of the report also provides the diagnosed patient pool, its trends, and assumptions undertaken.
Examining the views of key opinion leaders from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
Key Findings
- As per the research conducted by Pound et al. (2002), in the US, the Incidence of Cryptococcus-specific fungal Pneumonia in HIV-infected patients was reported to be 30–35%.
- Research conducted by Drew et al. (2002) shows that the incidence of fungal Pneumonia in Bone marrow transplant patients was 12–56% in the US.
- According to a study by Rayens and Norris (2022), Aspergillus, Pneumocystis, and Candida infections in the US accounted for 76.3% of fungal infections diagnosed.
- According to the study conducted by Ruhnke et al. (2017), using local data and literature estimates of the incidence of fungal infections, about 12% of people in Germany suffer from a fungal infection each year.
- Based on the research conducted by Bitar et al. (2014), in France, among all the invasive fungal infections, candidemia accounted for the highest proportion of cases (43.4%).
Fungal Pneumonia Market Outlook
With increasing numbers of immune-compromised patients with malignancy, hematologic disease, and HIV and those receiving immunosuppressive drug regimens for managing organ transplantation or autoimmune inflammatory conditions, the incidence of fungal infections has dramatically increased over recent years. While traditionally, antifungal therapy was limited to the use of amphotericin B, flucytosine, and a handful of clinically available azole agents; current pharmacologic treatment options include potent new azole compounds with extended antifungal activity, lipid forms of amphotericin B, and newer antifungal drugs, including the echinocandins. In March 2023, the US FDA approved REZZAYO (rezafungin for injection) to treat candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Fungal Pneumonia Drug Chapters
Fungal Pneumonia Marketed Drugs
REZZAYO: Cidara Therapeutics/Melinta Therapeutics
REZZAYO (rezafungin for injection) is an echinocandin antifungal indicated in patients 18 or older with limited or no alternative options for treating candidemia and invasive candidiasis. Approval of this indication is based on limited clinical safety and efficacy data. REZZAYO (rezafungin for injection) is a novel once-weekly echinocandin approved in the United States for treating candidemia and invasive candidiasis in adults.
The FDA approval of once-weekly REZZAYO was based on clinical data from Cidara’s global ReSTORE Phase II trial and supported by the STRIVE Phase II clinical trial and extensive non-clinical development program. In clinical studies, REZZAYO, dosed once weekly, met the FDA and EMA primary endpoints, demonstrating statistical non-inferiority versus caspofungin, a current once-daily standard of care. EMA regulatory decision for rezafungin is expected in year-end of 2023.
Fungal Pneumonia Emerging Drugs
Research and development efforts by researchers and pharmaceutical companies aim to explore novel treatment options for Fungal Pneumonia. These potential treatments target the underlying mechanisms of the condition, offering hope for improved management and relief for individuals affected by Fungal Pneumonia.
The Fungal Pneumonia market dynamics are expected to change, primarily due to increased healthcare spending worldwide. Fungal Pneumonia Market players such as Pfizer, F2G Ltd, Scynexis Inc, Matinas Biopharma, and others are actively involved in developing Fungal Pneumonia treatments.
Fosmanogepix (APX001): Pfizer
Fosmanogepix (FMGX, APX001) is a first-in-class, intravenous, and oral antifungal product candidate currently in clinical development for treating invasive fungal infections.
Fosmanogepix is currently in Phase II clinical trials evaluating the safety and efficacy of both intravenous (IV) and oral formulations for the treatment of patients with life-threatening invasive fungal infections caused by molds, yeasts, and rare molds (e.g., Aspergillus spp, Candida spp, including Candida auris, Fusarium spp., and Scedosporium spp). Fosmanogepix has demonstrated broad-spectrum activity in vitro and wide distribution to various tissues, including the brain, lung, kidney, and eye. With both IV and oral formulations in development, Fosmanogepix may allow for the transition from IV to oral, thus potentially enabling the continuation of treatment outside the hospital to benefit patients.
Note: Detailed emerging therapies assessment will be provided in the final report...
Fungal Pneumonia Market Segmentation
DelveInsight’s ‘Fungal Pneumonia Market Insights, Epidemiology, and Market Forecast – 2032’ report provides a detailed outlook of the current and future Fungal Pneumonia market, segmented within countries and by therapies. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Fungal Pneumonia market size by countries
The total Fungal Pneumonia market size is analyzed for individual countries of the 7MM (the United States Market, EU4 (Germany, France, Italy, and Spain) and the UK market, and Japan market). The United States accounted for a larger portion of the 7MM market for Fungal Pneumonia in 2022 due to the high incidence of the condition and the higher cost of treatments. This dominance is predicted to continue with the potential early entry of new products.
Fungal Pneumonia Market Size by Therapies
Fungal Pneumonia Market Size by Therapies is categorized as Antifungals, Antibiotics, surgery, and others. Fosmanogepix (APX001) is a drug in the developmental pipeline of Pfizer and is likely to get approval.
Note: Detailed market segment assessment will be provided in the final report...
Fungal Pneumonia Drugs Uptake
This section focuses on the sales uptake of potential Fungal Pneumonia drugs that have recently launched or are anticipated to be launched in the Fungal Pneumonia market between 2019 and 2032. It estimates the market penetration of Fungal Pneumonia drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Fungal Pneumonia market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Fungal Pneumonia...
Fungal Pneumonia Market Access and Reimbursement
DelveInsight’s ‘Fungal Pneumonia Market Insights, Epidemiology, and Market Forecast – 2032’ report provides a descriptive overview of the market access and reimbursement scenario of Fungal Pneumonia.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Fungal Pneumonia market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Fungal Pneumonia domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Fungal Pneumonia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Fungal Pneumonia unmet needs.
Fungal Pneumonia: KOL Insights
- DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Internal Medicine/Infectious Diseases/Academia, Division of Infectious Diseases, Duke University Medical Center, USA, Instituto de investigación Biomédica Ramón y Cajal (IRYCIS), Madrid, Spain, Department of Pharmacy, Duke University Hospital, Durham, NC, USA, Public Health England North West Health Protection Team (Greater Manchester), UK, and others.
- “While the incidence of pulmonary fungal infections has increased over the years, advances in diagnostic techniques and treatments have improved. Despite these advances, patient outcomes remain poor owing to a lack of early infection identification.”
Note: Detailed assessment of KOL Views will be provided in the full report on Fungal Pneumonia...
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Fungal Pneumonia Market using various Competitive Intelligence tools, including SWOT analysis, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Fungal Pneumonia Pipeline Development Activities
The report provides insights into Fungal Pneumonia Clinical Trials within Phase II and III stages. It also analyses Fungal Pneumonia companies involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Fungal Pneumonia therapies.
Fungal Pneumonia Report Insights
- Fungal Pneumonia Patient Population
- Fungal Pneumonia Therapeutic Approaches
- Fungal Pneumonia Pipeline Analysis
- Fungal Pneumonia Market Size and Trends
- Fungal Pneumonia Market Opportunities
- Impact of Upcoming Fungal Pneumonia Therapies
Fungal Pneumonia Report Key Strengths
- 10 Years Forecast
- The 7MM Coverage
- Fungal Pneumonia Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Fungal Pneumonia Market
- Fungal Pneumonia Drugs Uptake
Fungal Pneumonia Report Assessment
- Fungal Pneumonia Current Treatment Practices
- Fungal Pneumonia Unmet Needs
- Fungal Pneumonia Pipeline Product Profiles
- Fungal Pneumonia Market Attractiveness
- Fungal Pneumonia Market Drivers
- Fungal Pneumonia Market Barriers
Key Questions Answered In The Fungal Pneumonia Market Report
- What are the key findings of the market across the 7MM, and what country will have the largest Fungal Pneumonia market size during the forecast period (2023–2032)?
- What are the major causes of Fungal Pneumonia, and how is it treated?
- At what CAGR is the Fungal Pneumonia market, and its epidemiology is expected to grow in the 7MM forecast period (2023–2032)?
- How would the unmet needs impact the Fungal Pneumonia market dynamics and subsequently influence the analysis of related trends?
- What would be the forecasted patient pool of Fungal Pneumonia in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan?
- What are the current treatment guidelines and options for Fungal Pneumonia in the US, Europe, and Japan?
- What are the latest advancements in novel therapies, targets, mechanisms of action, and technologies being developed to address the limitations of existing therapies for Fungal Pneumonia?
- How many companies are currently engaged in the development of therapies for the treatment of Fungal Pneumonia?