Gastroesophageal Adenocarcinoma Market
- Japan accounted for the highest number of Incident Cases of Gastroesophageal adenocarcinoma in 2023.
- In November 2023, the Food and Drug Administration approved KEYTRUDA (pembrolizumab, Merck) with fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of adults with locally advanced unresectable or metastatic HER2-negative gastroesophageal adenocarcinoma.
- In October 2023, ALX Oncology unveiled positive interim data from the Phase II ASPEN-06 regarding Evorpacept in HER2-positive gastric/gastroesophageal junction cancer. The company look forward to report the final analysis from this study in Q2 2024 and plan to initiate the Phase III portion of ASPEN-06 in late 2024.
- In June 2023, ALX Oncology Received Orphan Drug Designation from the European Commission for Evorpacept for the Treatment of Patients with Gastroesophageal adenocarcinoma.
- In October 2023, Merck announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending approval of KEYTRUDA, Merck’s anti-PD-1 therapy, in combination with fluoropyrimidine- and platinum-containing chemotherapy, for the first-line treatment of locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma in adults whose tumors express PD-L1.
DelveInsight's “Gastroesophageal Adenocarcinoma Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of Gastroesophageal adenocarcinoma epidemiology, market, and clinical development in Gastroesophageal adenocarcinoma. In addition to this, the report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the Gastroesophageal Adenocarcinoma market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Gastroesophageal Adenocarcinoma market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted Gastroesophageal Adenocarcinoma market size from 2020 to 2034 in 7MM. The report also covers current Gastroesophageal Adenocarcinoma treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Gastroesophageal Adenocarcinoma Understanding and Treatment Algorithm
Gastroesophageal Adenocarcinoma Overview and Diagnosis
Gastroesophageal junction adenocarcinoma is a rare type of cancer of the esophagus, the tube that connects your mouth and stomach. It starts in the gastroesophageal (GE) junction, the area where the esophagus and stomach join together. The cancer grows from cells that make mucus. Gastroesophageal cancer can manifest as chest pressure or burning due to acid reflux, unintended weight loss, difficulty swallowing, particularly with solid, dry foods which worsens progressively, and symptoms of anemia such as pale skin, fatigue, and shortness of breath. Additionally, it may lead to a hoarse voice.
Diagnosing esophageal cancer involves endoscopy as the primary method, along with tests like upper GI series, CT scans, and PET scans to assess the extent of the disease. These tests help determine the presence and spread of cancer, guiding treatment decisions.
The Gastroesophageal Adenocarcinoma report provides an overview of Gastroesophageal Adenocarcinoma pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report...
Gastroesophageal Adenocarcinoma Treatment
Treatment options and recommendations depend on several factors, including the type and stage of cancer, possible side effects, the patient’s preferences, and the overall health of an individual. The most common treatments for Gastroesophageal Adenocarcinoma are:
- Esophagectomy surgery
- Esophageal Dilation
- radiotherapy
- chemotherapy
- chemotherapy with radiotherapy (chemoradiotherapy)
- targeted cancer drugs
Gastroesophageal Adenocarcinoma Epidemiology
The Gastroesophageal Adenocarcinoma epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total Incident Cases of Gastroesophageal adenocarcinoma (Both Esophageal and gastric included), Stage-specific Cases of Gastroesophageal adenocarcinoma, Gender-specific Cases of Gastroesophageal adenocarcinoma, Grade-specific Cases of Gastroesophageal adenocarcinoma, and total HER2 Positive Cases of Gastroesophageal adenocarcinoma in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
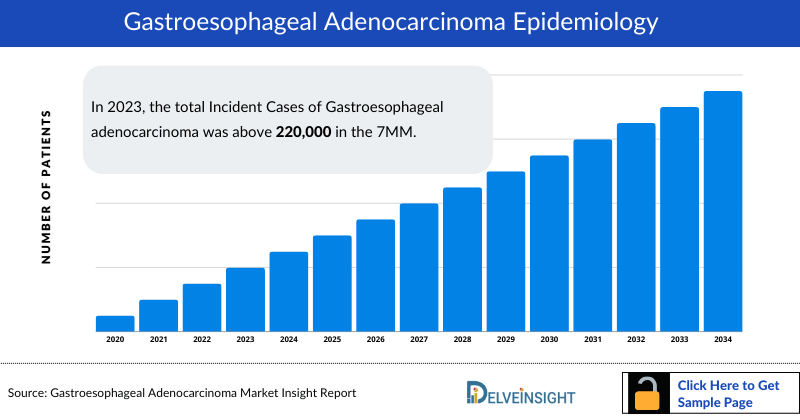
- In 2023, the total Incident Cases of Gastroesophageal adenocarcinoma was above 220,000 in the 7MM.
- Among the 7MM, Japan accounted for the highest number of incident cases of gastroesophageal adenocarcinoma in 2023.
- Among EU4 and the UK, Germany accounts for the highest and Spain for the lowest number of Incident Cases of Gastroesophageal adenocarcinoma.
- Gastroesophageal adenocarcinoma was observed to more common in males than females
- In the US, Stage IV accounted for the highest number of incident cases of Gastroesophageal adenocarcinoma, while in Japan, highest cases were observed in Stage I.
Gastroesophageal Adenocarcinoma Drug Chapters
The drug chapter segment of the Gastroesophageal Adenocarcinoma report encloses a detailed analysis of Gastroesophageal Adenocarcinoma marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Gastroesophageal Adenocarcinoma pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Marketed Drugs
KEYTRUDA (pembrolizumab): Merck
On May 5, 2021, the FDA granted accelerated approval to pembrolizumab (brand name KEYTRUDA) in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic HER2 positive gastroesophageal adenocarcinoma. Common side effects include fatigue, musculoskeletal pain, decreased appetite, itchy skin (pruritus), diarrhea, nausea, rash, fever (pyrexia), cough, difficulty breathing (dyspnea), constipation, pain, and abdominal pain. It is an IgG4 isotype antibody that blocks a protective mechanism of cancer cells, allowing the immune system to destroy them. It targets the programmed cell death protein 1 (PD-1) receptor of lymphocytes.
LONSURF (trifluridine/ tipiracil): Taiho Pharmaceuticals
In February 2019, FDA approved trifluridine/ tipiracil tablets, a fixed combination of trifluridine, a nucleoside metabolic inhibitor, and tipiracil, a thymidine phosphorylase inhibitor, for adult patients with metastatic gastroesophageal adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, platinum, either a taxane or irinotecan and if appropriate, HER2/neu-targeted therapy. The most common side effects of LONSURF when used include low blood counts, tiredness and weakness, nausea, decreased appetite, diarrhea, vomiting, stomach-area (abdominal) pain, and fever.
|
Table 1: Comparison of Key Marketed drugs | ||||
|
Drug name |
Company |
RoA |
MoA |
Approval |
|
KEYTRUDA |
Merck |
Intravenous |
Programmed cell death protein (PD-1) inhibitor |
US: 2021 |
|
LONSURF |
Taiho Pharmaceuticals |
Oral |
Fixed Combination of Nucleoside metabolic inhibitor and thymidine phosphorylase inhibitor |
US: 2019 |
Note: Detailed current therapies assessment will be provided in the full report of Gastroesophageal Adenocarcinoma...
Emerging Drugs
Sacituzumab Tirumotecan (MK-2870): Merck/Sichuan Kelun-Biotech
Sacituzumab tirumotecan is an investigational antibody-drug conjugate that consists of an antibody-targeting trophoblast cell-surface antigen 2 (TROP2) linked to a belotecan-derived payload. Sacituzumab tirumotecan is being developed as monotherapy and/or in combination with KEYTRUDA. It is being developed in collaboration with Sichuan Kelun-Biotech. Currently, it is in Phase III for treatment in 3L+ Advanced/Metastatic Gastroesophageal adenocarcinoma.
Evorpacept (ALX148): ALX Oncology
In advanced HER2-positive gastric and gastroesophageal junction cancer patients, ALX148, a CD47 blocker developed by ALX Oncology, showed enhanced treatment response when used as a second- or third-line therapy alongside trastuzumab, ramucirumab, and paclitaxel. This improvement was observed compared to treatment with trastuzumab, ramucirumab, and paclitaxel alone. These findings were consistent even among patients previously treated with fam-trastuzumab deruxtecan-nxki (ENHERTU) and checkpoint inhibitor therapy. Currently, ALX148 is in Phase II of its development process.
|
Table 2: Comparison of key emerging drugs | |||||
|
Drug name |
Company |
RoA |
MoA |
Phase |
Special Status |
|
Sacituzumab Tirumotecan (MK-2870) |
Merck/ Sichuan Kelun-Biotech |
Intravenous |
RS7, a humanized antibody, targets TROP-2 cancer cells, releasing SN-38 internally to trigger DNA damage-induced apoptosis |
III |
NA |
|
Evorpacept (ALX148) |
ALX Oncology |
Intravenous |
Anti-CD47 blocker |
II |
EU: Orphan Drug Designation |
Note: Detailed emerging therapies assessment will be provided in the final report...
Drug Class Insights
Drug development for gastroesophageal adenocarcinoma involves various approaches, such as PD-1 inhibitors, CD47 blockers, and a humanized antibody RS7, which targets TROP-2 cancer cells by releasing SN-38 internally to induce apoptosis through DNA damage.
A protein found on T cells (a type of immune cell) that helps keep the body’s immune responses in check. When PD-1 is bound to another protein called PD-L1, it helps keep T cells from killing other cells, including cancer cells. Some anticancer drugs, called immune checkpoint inhibitors, are used to block PD-1. When this protein is blocked, the “brakes” on the immune system are released and the ability of T cells to kill cancer cells is increased. There is increasing evidence that immunotherapy (programmed cell death-1 (PD-1) inhibitor) combined with chemotherapy is superior to chemotherapy alone in neoadjuvant therapy for patients with previously untreated, unresectable advanced, or metastatic esophageal adenocarcinoma (EAC)/gastric/gastroesophageal junction.
Gastroesophageal adenocarcinoma Market Outlook
Incidence rates for Gastroesophageal adenocarcinoma cancer are rising rapidly worldwide possibly due to economic development and demographic changes. Therefore, increased attention has been paid to the prevention, diagnosis, and treatment of Gastroesophageal adenocarcinoma cancer. Although there are discrepancies in the treatment strategy between Asian and Western countries, surgery remains the mainstay of treatment for Gastroesophageal adenocarcinoma cancer. Recent developments in perioperative multidisciplinary treatment may lead to better therapeutic effects, higher complete resection rates, and better control of residual diseases, thus resulting in prolonged prognosis. There are several types of chemotherapeutic agents such as ADC (antibody-drug conjugates), immunotherapies, vaccines, and a variety of targeted therapies like anti-HER2+, anti-PD-1 agents, etc.
Key players, such as Merck, ALX Oncology, and others are evaluating their candidates in different stages of clinical development. They aim to investigate their products for the treatment of Gastroesophageal adenocarcinoma.
Gastroesophageal adenocarcinoma Drugs Uptake
Current findings suggest ALX148, when combined with anticancer drugs like HERCEPTIN and KEYTRUDA for solid tumors, demonstrates generally good tolerance with manageable adverse effects. Consequently, ALX Oncology's Phase II development of Evorpacept for gastroesophageal adenocarcinoma appears promising, given its positive outcomes. Also in October 2023, ALX Oncology reported positive Interim Phase 2 ASPEN-06 Clinical Trial Results of Evorpacept for the Treatment of Advanced HER2-Positive Gastric Cancer. The data highlight the drug’s potential as a first-in-class foundational immunotherapy.
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Gastroesophageal adenocarcinoma Cancer Activities
The report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Gastroesophageal adenocarcinoma emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Medical Oncologists, Chief of the Thoracic Service at the Memorial Sloan Kettering Cancer Center, and Others.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as MD Anderson Cancer Center, Texas, UT Southwestern Medical Center in Dallas, Cancer Research UK Barts Centre in London, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Gastroesophageal adenocarcinoma market trends.
|
KOL Views |
|
“Patients with HER2-positive gastric/GEJ cancer need tolerable and effective treatments, particularly in the second-line and later settings where resistance to HER2-directed therapy may have developed” |
|
“We are greatly encouraged by the exciting data that continue to emerge from ASPEN-01 and are pleased to announce the first patient dosed in ASPEN-06, This milestone is an important step towards establishing Evorpacept as a unique CD47 blocker that may be used in combination with other anti-cancer drugs for difficult-to-treat solid tumors, such as gastric/GEJ cancer, where more treatment options are desperately needed to improve disease outcomes.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, descriptive overview of Gastroesophageal adenocarcinoma, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Gastroesophageal adenocarcinoma market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the Gastroesophageal adenocarcinoma market.
Gastroesophageal adenocarcinoma Report Insights
- Patient Population
- Therapeutic Approaches
- Gastroesophageal adenocarcinoma Pipeline Analysis
- Gastroesophageal adenocarcinoma Market Size and Trends
- Existing and future Market Opportunity
Gastroesophageal adenocarcinoma Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Gastroesophageal adenocarcinoma Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
Gastroesophageal adenocarcinoma Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the historical and forecasted Gastroesophageal adenocarcinoma patient pool/patient burden in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What was the Gastroesophageal adenocarcinoma total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Which combination treatment approaches will have a significant impact on Gastroesophageal adenocarcinoma drug treatment market size?
- Is there any unexplored patient setting that can open the window for growth in the future?
- Which class is going to be the largest contributor to market size in 2034?
- What are major changes in new treatment guidelines from ASCO or ESMO and what impact on future treatment landscape?
- What are the pricing variations among different geographies for approved therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of Gastroesophageal adenocarcinoma?
- How many companies are developing therapies for the treatment of Gastroesophageal adenocarcinoma?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Gastroesophageal adenocarcinoma Market.
- Insights on patient burden/disease Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Patient-based forecast model which uses bottom-up forecasting techniques is accepted as a gold standard in pharma forecasting.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy