Generalized Pustular Psoriasis Market
- Generalized Pustular Psoriasis is a subtype of pustular psoriasis characterized by sudden, repeated episodes of high-grade fever, generalized erythematous pustular rashes, painful and occasionally disfiguring cutaneous manifestations with sepsis-like.
- While Generalized pustular psoriasis usually affects adults (40-50), children can develop it, often due to genetic changes, or mutations. The most common mutations linked to Generalized pustular psoriasis occur in the IL36RN gene, which leads to unregulated inflammatory cytokine production, causing inflammation in the skin.
- Generalized pustular psoriasis may be more common in women than in men, with female-to-male ratios ranging from 2:1 to 1.2:1. Hormonal fluctuations during pregnancy and menstruation are known triggers of Generalized pustular psoriasis and may predispose women to the condition. One subtype of Generalized pustular psoriasis is pustular psoriasis of pregnancy.
- The therapeutic approach to Generalized Pustular Psoriasis in the US, EU4, and the UK predominantly relies on supportive therapies such as topical treatments, systemic medications, biologics, and phototherapy, mainly because of the absence of widely approved treatment protocols. Currently, only one therapy (SPEVIGO) is approved in the US. In contrast, Japan boasts a more diverse therapeutic landscape for Generalized Pustular Psoriasis, along with supportive treatment regimens.
- Although now significant development is taking place in improving the treatment landscape of Generalized Pustular Psoriasis, for instance in March 2024, the US Food and Drug Administration (FDA) expanded approval for Boehringer’s SPEVIGO to include the treatment of Generalized pustular psoriasis in adults and pediatric patients aged 12 and above weighing ≥40 kg. Previously approved for treatment of Generalized pustular psoriasis flares in adults, this expanded approval addresses the need for acute and chronic treatment for Generalized pustular psoriasis patients.
- The pipeline for Generalized pustular psoriasis remains robust, with major pharmaceutical players such as AnaptysBio, Takeda, Johnson & Johnson, and others deeply engaged in advancing clinical research and development efforts to enhance treatment options for patients afflicted by this condition.
- AnaptysBio announced the positive result of Phase III clinical trial (GEMINI-1) of Imsidolimab for the treatment of Generalized pustular psoriasis and the company is now planning to to out-license imsidolimab in 2024 and submit comprehensive data abstract to H2 2024 medical meeting.
Request for Sample Page @ Generalized Pustular Psoriasis Market Report
DelveInsight's “Generalized Pustular Psoriasis Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Generalized Pustular Psoriasis, historical and forecasted epidemiology as well as the Generalized Pustular Psoriasis market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Generalized Pustular Psoriasis market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Generalized Pustular Psoriasis market size from 2020 to 2034. The report also covers current Generalized Pustular Psoriasis treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
The US, EU4 (Germany, France, Italy, and Spain) and UK, Japan |
|
Generalized Pustular Psoriasis Market |
|
|
Generalized Pustular Psoriasiss Market Size | |
|
Generalized Pustular Psoriasis Companies |
AbbVie, Boehringer Ingelheim, UCB Pharma, and others. |
|
Generalized Pustular Psoriasis Epidemiology Segmentation |
|
Generalized Pustular Psoriasis Treatment Market
Generalized Pustular Psoriasis Overview, Country-Specific Treatment Guidelines and Diagnosis
Generalised pustular psoriasis, also known as von Zumbusch psoriasis is a rare, severe form of pustular psoriasis. It is characterised by recurrent flares of widespread sterile pustules with erythematous, painful skin. Generalised pustular psoriasis can be associated with systemic inflammation including fevers and/or hepatic, gastrointestinal, musculoskeletal, renal, or pulmonary involvement. The most frequent causes for Generalised pustular psoriasis are genetic mutations, infections, stress, corticosteroid treatment withdrawal, and pregnancy.
Generalised pustular psoriasis can be a diagnostic challenge due to its rarity, the condition is often accompanied by constitutional symptoms, such as fever, chills, fatigue, and decreased appetite. Complications such as infection and organ failure can become life-threatening.
The Generalised pustular psoriasis report provides an overview of Generalised pustular psoriasis pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report...
Generalized Pustular Psoriasis Treatment
Treatment guidelines for Generalised pustular psoriasis are not well-established, and only one FDA-approved GPP-specific medication, SPEVIGO, is currently approved for use in the US. Recently, the European Commission has approved Spevigo for use in adults with GPP flares in the European Union (EU) countries. Other treatments include additional inhibitors of the IL-36 pathway, IL-17 inhibitors, IL-23 inhibitors, TNF-α inhibitors, retinoids, calcineurin inhibitors, anti-folate therapy, and PDE4 inhibitors.
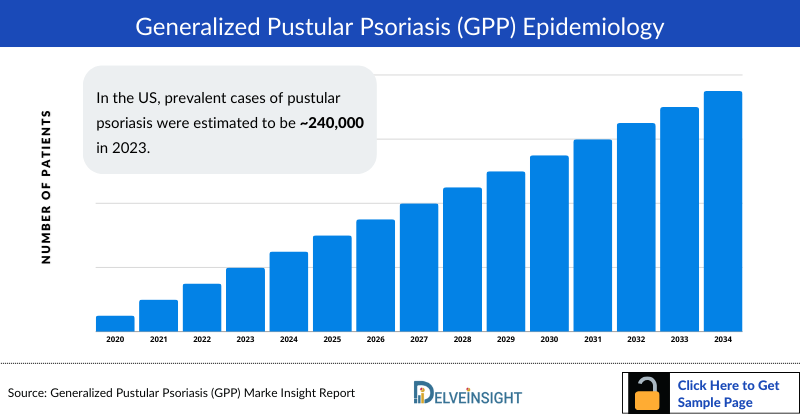
Generalized Pustular Psoriasis Epidemiology
The Generalized Pustular Psoriasis epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Generalized Pustular Psoriasis epidemiology is segmented with detailed insights into total prevalent cases of Psoriasis, total prevalent cases of pustular psoriasis, total prevalent cases of generalized pustular psoriasis, gender-specific diagnosed prevalent Cases of Generalized Pustular Psoriasis, age-specific diagnosed prevalent cases of Generalized Pustular Psoriasis, Severity-specific Diagnosed Prevalent Cases.
- According to the findings, females tend to be more likely to present with Generalized pustular psoriasis than males.
- In the US, prevalent cases of pustular psoriasis were estimated to be ~240,000 in 2023.
- Generalized pustular psoriasis patients are frequently categorized into two groups: mild-to-moderate, and moderate-to-severe psoriasis, In the US, severity-specific cases of Generalized pustular psoriasis were highest in moderate to severe type in 2023.
Discover crucial insights with our Generalized Pustular Psoriasis Epidemiology Forecast 2032. Stay ahead in healthcare innovation.
Generalized Pustular Psoriasis Drug Chapters
The drug chapter segment of the Generalized Pustular Psoriasis report encloses a detailed analysis of Generalized Pustular Psoriasis marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Generalized Pustular Psoriasis pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Generalized Pustular Psoriasis Marketed Drugs
SPEVIGO (Spesolimab): Boehringer Ingelheim
Spesolimab-sbzo is a humanized monoclonal immunoglobulin G1 antibody that inhibits interleukin-36 (IL-36) signaling by specifically binding to the IL36R. Binding of spesolimab-sbzo to IL36R prevents the subsequent activation of IL36R by its ligands (IL-36 α, β, and γ) and downstream activation of pro-inflammatory and profibrotic pathways. SPEVIGO (spesolimab) is the only drug approved for the treatment of generalized pustular psoriasis flares in adults, it was approved in 2022. The drug has received breakthrough therapy designation and Priority review designation in the US.
|
Therapy Name |
Company Name |
ROA |
MOA |
Any Special Status |
Approval year |
|
SKYRIZI |
AbbVie |
Subcutaneous injections |
Interleukin-23 subunit p19 inhibitors |
NA |
2019 (approved in Japan) |
|
SPEVIGO |
Boehringer Ingelheim |
Intravenous infusion |
interleukin-23 (IL-23) inhibitor |
breakthrough therapy designation/ Priority review designation |
2022 (only approved in the US and EU4 and the UK) |
|
BIMZELX |
UCB |
Subcutaneous injections |
interleukin 17A and interleukin 17F inhibitors |
NA |
2022 (approved in Japan) |
Note: Detailed current therapies assessment will be provided in the full report of Generalized Pustular Psoriasis...
Generalized Pustular Psoriasis Emerging Drugs
TAK-279: Takeda
TAK-279 is a highly selective, oral allosteric tyrosine kinase 2 (TYK2) inhibitor in late-stage development, with approximately 1.3 million-fold greater selectivity for TYK2 as compared with JAK1. TAK-279 has the potential to become an important treatment option in multiple immune-mediated inflammatory diseases.
Currently, it is in Phase III of its clinical development for the treatment of Generalized pustular psoriasis.
JNJ-77242113 (JNJ-2113): Johnson & Johnson
JNJ-2113 is the first targeted oral peptide designed to block the IL-23 receptor, which underpins the inflammatory response in moderate-to-severe plaque psoriasis and other IL-23-mediated diseases. JNJ-2113 binds to the IL-23 receptor with single-digit picomolar affinity and demonstrates potent, selective inhibition of IL-23 signaling in human T cells. Currently, it is in Phase III of its clinical development for the treatment of Generalized pustular psoriasis.
JNJ-2113 was jointly discovered and is being developed under the license and collaboration agreement between Protagonist Therapeutics and Johnson & Johnson. Johnson & Johnson retains exclusive worldwide rights to develop JNJ2113 in Phase II clinical trials and beyond and to commercialize compounds derived from the research conducted according to the agreement against a broad range of indications.
|
Therapy Name |
Company Name |
ROA |
MOA |
Phases |
Any Special Status |
|
Imsidolimab |
AnaptysBio |
Intravenous |
Interleukin 36 receptor antagonists |
III(Completed) |
ODD |
|
TAK-279 |
Takeda |
Oral |
TYK2 kinase inhibitors |
III |
NA |
|
JNJ-2113 |
Johnson & Johnson |
Oral |
Interleukin-23 receptor antagonists |
III |
NA |
Note: Detailed emerging therapies assessment will be provided in the final report...
Generalized Pustular Psoriasis Market Outlook
Key players, such as AnaptysBio, Takeda, Johnson & Johnson, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Generalized Pustular Psoriasis.
Generalized Pustular Psoriasis Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Generalized Pustular Psoriasis Activities
The report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Generalized Pustular Psoriasis emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of Generalized Pustular Psoriasis. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Generalized Pustular Psoriasis Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Generalized Pustular Psoriasis, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Generalized Pustular Psoriasis market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Generalized Pustular Psoriasis market.
Generalized Pustular Psoriasis Report Insights
- Generalized Pustular Psoriasis Patient Population
- Generalized Pustular Psoriasis Therapeutic Approaches
- Generalized Pustular Psoriasis Pipeline Analysis
- Generalized Pustular Psoriasis Market Size and Trends
- Existing and future Market Opportunity
Generalized Pustular Psoriasis Report Key Strengths
- Ten Years Forecast
- 7MM Coverage
- Generalized Pustular Psoriasis Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Generalized Pustular Psoriasis Drugs Uptake
- Key Generalized Pustular Psoriasis Market Forecast Assumptions
Generalized Pustular Psoriasis Report Assessment
- Current Generalized Pustular Psoriasis Treatment Practices
- Generalized Pustular Psoriasis Unmet Needs
- Generalized Pustular Psoriasis Pipeline Product Profiles
- Generalized Pustular Psoriasis Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Generalized Pustular Psoriasis Market Drivers
- Generalized Pustular Psoriasis Market Barriers
FAQs
- What is the growth rate of the 7MM Generalized Pustular Psoriasis treatment market?
- What was the Generalized Pustular Psoriasis total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of Generalized Pustular Psoriasis?
- How many companies are developing therapies for the treatment of Generalized Pustular Psoriasis?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy Generalized Pustular Psoriasis Market Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Generalized Pustular Psoriasis Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.