GM2 Gangliosidosis Market
- The GM2 Gangliosidosis Market is expected to witness moderate growth during the forecast period (2024–2034). The increase in market size is a direct consequence of increased awareness about the disease among doctors, increased R&D efforts to cure genetic disorders, improved diagnostic practices, and emerging therapeutic approaches.
- While several approaches have been tested for the development of specific treatments for GM2 Gangliosidosis, there is currently an absence of any US FDA-approved drug for the condition. Due to this unmet need in the GM2 Gangliosidosis treatment pharmaceutical companies are further driven to develop a therapeutic agent capable of managing symptoms and the disease.
- To propel the market in the coming years, several GM2 Gangliosidosis Companies like Azafaros A.G., IntraBio, and Sio Gene Therapeutics are developing their assets like AZ-3102 (nizubaglustat) and IB1001 for GM2 Gangliosidosis. There are some ongoing developments in the field of gene therapy as well, with investigational treatments like AXO-AAV-GM2 showing promise in the trials. Due to the anticipated approval of a few emerging therapies which are under development in the coming years the overall GM2 Gangliosidosis therapeutics market is expected to grow at a significant CAGR over the forecast period (2024–2034).
Request for unlocking the CAGR of the "GM2 Gangliosidosis Drug Market”
DelveInsight’s comprehensive report titled “GM2 Gangliosidosis Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of GM2 Gangliosidosis. The report presents historical and projected epidemiological data covering total incident cases of GM2 Gangliosidosis, mutation-specific incident cases of GM2 Gangliosidosis, and treated cases of GM2 Gangliosidosis. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing treatment practices and unmet medical requirements in GM2 Gangliosidosis. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
GM2 Gangliosidosis Treatment Market: Overview
GM2 Gangliosidosis is a group of inherited disorders (Tay-Sachs disease, Sandhoff disease, and AB variant) caused by excessive accumulation of GM2 ganglioside into the lysosome due to mutations on the genes encoding for the β-hexosaminidases subunits or the GM2 activator protein. GM2 gangliosidosis progressively destroys nerve cells in the brain and spinal cord. Tay-Sachs disease (TSD) is caused by a mutation to the HEXA gene, Sandhoff disease (SD) is caused by a mutation to the HEXB gene, and GM2 activator deficiency (AB variant) occurs due to a mutation of the GM2A gene.
TSD, SD, and AB variants are caused by mutation in different genes but the neurological compromise is similar among these three disorders. Symptoms include seizures, hypotonia, motor regression, psychotic episodes, dysphagia, cerebellar, ataxia, etc. GM2 Gangliosidosis can be diagnosed with the recognition of the clinical characteristics of these disorders. The specific diagnosis requires the determination of HexA and HexB activities by using artificial substrates.
GM2 Gangliosidosis Diagnosis and Treatment Algorithm
GM2 Gangliosidosis diagnosis is based on the determination of the activity of the enzyme β-hexosaminidase A and B in leukocytes or cultured fibroblasts. Decreased levels of hexosaminidases A and B are seen in patients with SD but solitarily decreased levels of hexosaminidases A are seen in TSD. It can be diagnosed with the recognition of clinical characteristics of these disorders, neuroimaging characterized by hyperdensity of basal ganglia, which can be accompanied by other changes in white matter and sometimes prominent, but non-specific, cerebellar atrophy.
Currently, there is no specific US FDA-approved treatment for GM2 Gangliosidosis. Several approaches have been tested for the development of specific treatments for GM2 Gangliosidosis. These strategies range from traditional enzyme replacement therapy alternatives to novel biotechnological tools such as CRISPR/Cas9 and prime editing. Various therapeutic strategies have been studied for GM2 gangliosidosis including enzyme replacement therapy, hematopoietic stem cell transplantation, pharmacological chaperones, substrate reduction therapy, and gene therapy.
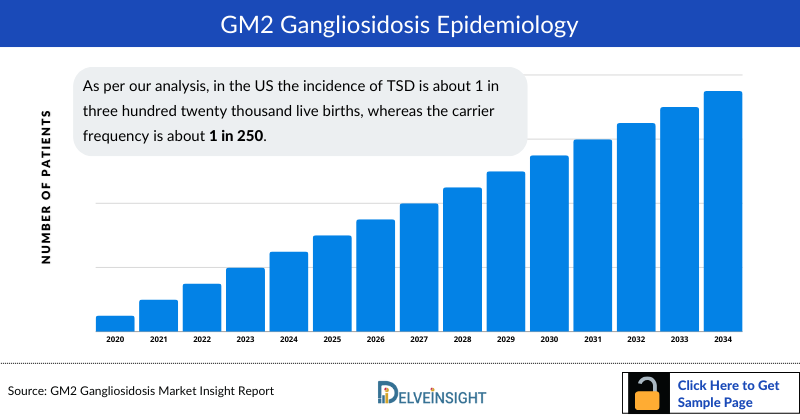
GM2 Gangliosidosis Epidemiology
The epidemiology section of the GM2 Gangliosidosis market size report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of GM2 Gangliosidosis. Additionally, the GM2 Gangliosidosis drug market report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
- As per analysis, around seventy individuals with GM2 Gangliosidosis were reported in the UK with around forty with Tay–Sachs disease, thirty with Sandhoff disease, and two with GM2 activator protein deficiency.
- As per our analysis, in the US the incidence of TSD is about 1 in three hundred twenty thousand live births, whereas the carrier frequency is about 1 in 250.
- As per our analysis, it is estimated that around 30 patients were presented with mutations in the HEXA gene, and around fifteen patients had defects in the HEXB gene. Of the Tay–Sachs patients, around twenty presented with the infantile form of the disease with the age of diagnosis ranging between 7 months and 3 years.
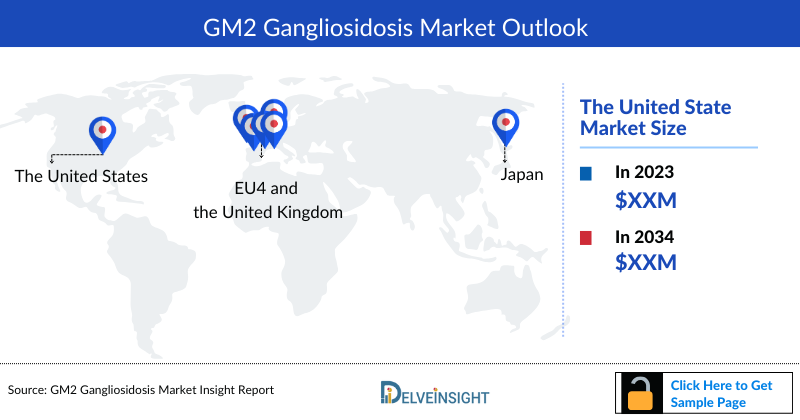
GM2 Gangliosidosis Market Outlook
At the moment, there is no specific US FDA-approved GM2 Gangliosidosis treatment. Despite this fact, several approaches have been rigorously tested for the development of specific treatments tailored to cure or manage GM2 Gangliosidosis. These strategies include a wide spectrum, ranging from traditional enzyme replacement therapy alternatives to cutting-edge biotechnological tools such as CRISPR/Cas9 and prime editing.
Gene therapy is being researched to potentially treat both Tay-Sachs and Sandhoff disease. Gene therapy aims to be a one-time treatment that could slow or stop disease progression by delivering working HEXA and HEXB genes into the cells using a viral vector. The viral vectors carrying the working HEXA and HEXB genes are delivered at the same time through a single injection. The goal is to restore how lysosomes function, which is to remove and prevent the build-up of toxic gangliosides.
Enzyme replacement therapy (ERT) is widely available for many LSDs including GM2 Gangliosidosis. Enzyme replacement therapy (ERT) is a therapeutic alternative in which the lysosomal enzymes can be uptake through endocytosis and delivered to the lysosomes. Hematopoietic Stem Cell Transplantation, since Hex can be exported to the extracellular space and cross-correct neighboring cells through an M6PR-mediated mechanism, the administration of hematopoietic stem cells (HSC), could provide sufficient amounts of the deficient enzyme in a natural o engineered-dependent manner.
Substrate reduction therapy (SRT) is a therapeutic strategy based on the partial inhibition of an enzyme involved in the synthesis of the accumulating substrate. One of the molecules that have been evaluated for this purpose is N-butyldeoxynojirimycin (NB-DNJ, also termed Miglustat or Zavesca). This is an iminosugar that inhibits the glucosylceramide synthase (GCS), which catabolizes the first step of glycosphingolipid synthesis such as GM2 gangliosides. With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the GM2 Gangliosidosis market in the 7MM is expected to change significantly during the study period 2020–2034.
Note: Detailed marketed therapies assessment will be provided in the final report.
GM2 Gangliosidosis Drug Chapters
GM2 Gangliosidosis Emerging Drugs
The GM2 Gangliosidosis pipeline is quite robust with many products available in the developmental stage. There are several key GM2 Gangliosidosis Companies involved in the development of promising products such as Azafaros A.G, IntraBio, and others.
- AZ-3102 (nizubaglustat): Azafaros A.G.
AZ-3102 is an orally available azasugar designed to reach the central nervous system and to interfere with the metabolism of glycosphingolipids through a unique and selective dual mode of action with equally potent inhibition of glucosylceramide synthase (GCS) and non-lysosomal glucosylceramidase (GbA2). With this new paradigm, AZ-3102 has the potential to reduce metabolite accumulation and ameliorate the function of the impaired lysosome. With its novel dual mode of action and ease of oral 2 administration, AZ-3102 holds the potential of a convenient life-long therapy that could mitigate the underlying pathophysiology of these diseases and favorably alter the patients’ clinical trajectory. AZ-3102 is under clinical development by Azafaros A.G. and is currently in Phase II for the treatment of GM2 Gangliosidosis.
- IB1001: IntraBio
IB1001 is an investigational gene therapy with a unique mechanism of action offering neuroprotective and symptomatic benefits for rare and common neurological disorders. IB1001 is initially being developed as a potential treatment for rare lysosomal storage disorders, including GM2 gangliosides. IB1001 met its primary and secondary endpoints and demonstrated a statistically significant and clear clinically meaningful improvement in symptoms, functioning, and quality of life for pediatric and adult patients with GM2 Gangliosidosis (Tay-Sachs and Sandhoff).
IB1001 was well-tolerated and no drug-related serious adverse events were reported, demonstrating a favorable risk-benefit profile for treating GM2 Gangliosidosis. IB1001 has been granted orphan drug designations from the US FDA and EMA. IB1001 is under clinical development by IntraBio and is currently in Phase II for GM2 Gangliosidosis.
Note: Detailed emerging therapies assessment will be provided in the final report.
GM2 Gangliosidosis Market Segmentation
DelveInsight’s ‘GM2 Gangliosidosis Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future GM2 Gangliosidosis market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
GM2 Gangliosidosis Market Size by Countries
The GM2 Gangliosidosis Market Size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2022, the United States held a significant share of the overall 7MM (Seven Major Markets) GM2 Gangliosidosis market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
GM2 Gangliosidosis Market Size by Therapies
GM2 Gangliosidosis Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is AZ-3102 (nizubaglustat): Azafaros A.G.
Note: Detailed market segment assessment will be provided in the final report.
GM2 Gangliosidosis Drugs Uptake
This section focuses on the sales uptake of potential GM2 Gangliosidosis drugs that have recently been launched or are anticipated to be launched in the GM2 Gangliosidosis market between 2020 and 2034. It estimates the market penetration of GM2 Gangliosidosis drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the GM2 Gangliosidosis drug market. The emerging GM2 Gangliosidosis therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the GM2 Gangliosidosis market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on GM2 Gangliosidosis.
GM2 Gangliosidosis Market Access and Reimbursement
DelveInsight’s ‘GM2 Gangliosidosis Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of GM2 Gangliosidosis.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current GM2 Gangliosidosis market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the GM2 Gangliosidosis domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or GM2 Gangliosidosis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the GM2 Gangliosidosis unmet needs.
GM2 Gangliosidosis KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Boston Children’s Hospital in the US, University Hospital Friuli Centrale, Udine, Italy, Great Ormond Street Hospital NHS Foundation Trust, London, United Kingdom, GM2 Gangliosidosis Registry, among others.
“GM2 Gangliosidosis should be diagnosed as early as possible as it is a fatal disease. Early diagnosis and preventive care can help in reducing symptoms and improving the quality of life.”
Note: Detailed assessment of KOL Views will be provided in the full report on GM2 Gangliosidosis.
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the GM2 Gangliosidosis Drug Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the GM2 Gangliosidosis drug market landscape and competitive dynamics.
GM2 Gangliosidosis Pipeline Development Activities
The GM2 Gangliosidosis pipeline segment offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for GM2 Gangliosidosis. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The GM2 Gangliosidosis pipeline segment covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging GM2 Gangliosidosis therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ GM2 Gangliosidosis Pipeline Report 2024
GM2 Gangliosidosis Market Forecast Report Insights
- Patient-based GM2 Gangliosidosis Market Forecasting
- Therapeutic Approaches
- GM2 Gangliosidosis Pipeline Analysis
- GM2 Gangliosidosis Market Size
- GM2 Gangliosidosis Market Trends
- GM2 Gangliosidosis Drug Market Opportunities
- Impact of Upcoming Therapies
GM2 Gangliosidosis Market Forecast Report Key Strengths
- 11 Years GM2 Gangliosidosis Market Forecast
- The 7MM Coverage
- GM2 Gangliosidosis Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed GM2 Gangliosidosis Drug Market
- GM2 Gangliosidosis Drugs Uptake
GM2 Gangliosidosis Treatment Market Report Assessment
- Current GM2 Gangliosidosis Treatment Practices
- GM2 Gangliosidosis Unmet Needs
- GM2 Gangliosidosis Pipeline Product Profiles
- GM2 Gangliosidosis Drug Market Attractiveness
Key Questions
- How common is GM2 Gangliosidosis?
- What are the key findings of GM2 Gangliosidosis epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available GM2 Gangliosidosis treatment?
- What are the disease risk, burden, and GM2 Gangliosidosis unmet needs?
- At what CAGR is the GM2 Gangliosidosis drug market and its epidemiology is expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the GM2 Gangliosidosis market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of GM2 Gangliosidosis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2024–2034)?
- How many companies are currently developing therapies for the GM2 Gangliosidosis treatment?
Ready to Dive Deeper? Purchase the Complete Report for in-depth Market Analysis by Clicking Here @ GM2 Gangliosidosis Treatment Market Report
Access Exclusive Data Now! Click here to Read More about the Related Articles @ Latest DelveInsight Blog