Golodirsen Sales Forecast Summary
Key Factors Driving Golodirsen Growth
Market Share Gains and New Patient Starts
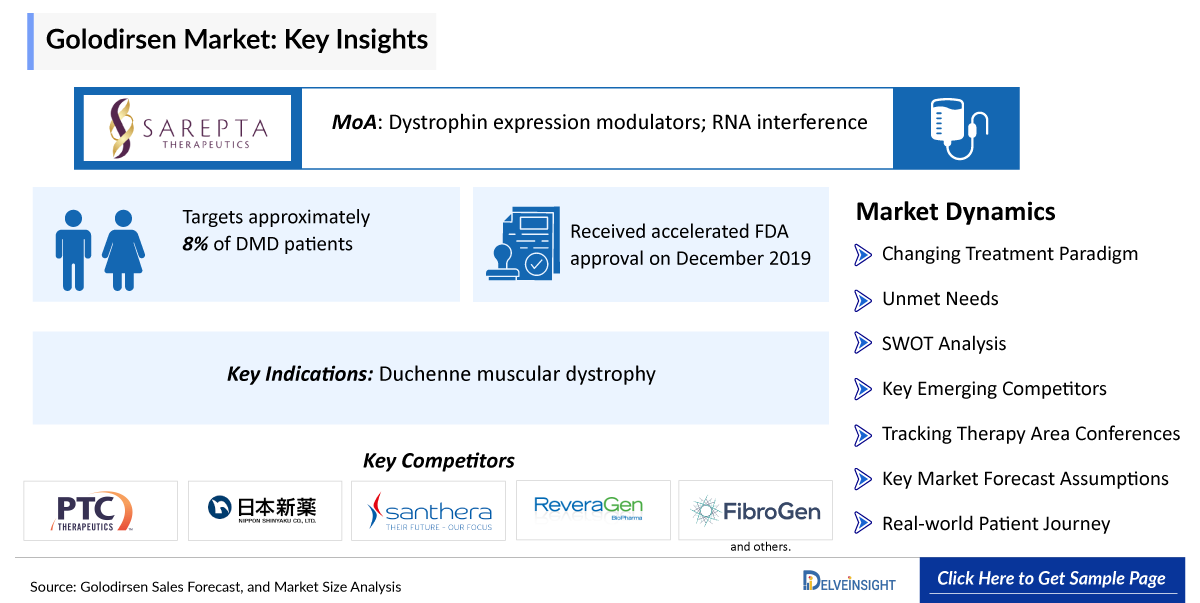
- Golodirsen (Vyondys 53) has established a strong position in the Duchenne muscular dystrophy (DMD) exon-skipping segment for patients with exon 53 skip-amenable mutations, representing ~8–10% of the DMD population.
- New patient starts are primarily driven by expanded genetic screening and earlier diagnosis, enabling identification of exon-53-eligible patients.
- Sarepta’s strong engagement with neuromuscular specialists, patient advocacy groups, and treatment centers continues to support steady uptake in the U.S. market.
Expansion Across Key Indications
- Primary indication – Duchenne Muscular Dystrophy: Golodirsen is approved for DMD patients with confirmed exon 53 mutations, designed to restore dystrophin expression via exon skipping.
- Ongoing studies are evaluating earlier intervention in younger patients, which could improve long-term functional outcomes and potentially support future label refinements.
- Unlike broad therapeutic areas, Golodirsen remains highly niche and mutation-specific, with expansion largely dependent on diagnostic penetration rather than new disease indications.
Geographic Expansion
- United States: Core commercial market with FDA approval and reimbursement pathways established for eligible patients.
- Japan: Approved under the brand name Vyondys 53, supporting moderate ex-U.S. expansion and contributing to international revenues.
- Europe and other regions: Limited uptake due to regulatory rejections or delays, though advocacy efforts continue for future market entry.
- Increased global awareness of genetic therapies and rising investment in rare disease infrastructure may improve long-term geographic reach.
New Indication Approvals
- Golodirsen received accelerated FDA approval based on its ability to increase dystrophin levels in muscle tissue.
- No additional disease indications have been approved beyond exon-53-amenable DMD, and future growth remains linked to confirmatory clinical outcomes rather than new therapeutic areas.
Strong DMD Outcome Momentum
- Clinical trials demonstrated statistically significant increases in dystrophin expression, supporting its biological activity.
- Long-term extension studies suggest slower decline in ambulatory and pulmonary function compared with historical natural history data.
- Real-world evidence from treatment centers indicates favorable tolerability and consistent safety, reinforcing physician confidence in chronic use.
Competitive Differentiation and Market Trends
- Precision genetic targeting: Golodirsen’s exon-53-specific mechanism differentiates it from corticosteroids and supportive care.
- Established exon-skipping franchise: Part of Sarepta’s broader dystrophin restoration portfolio (with eteplirsen and casimersen), enabling strong brand recognition in DMD.
- Rare disease market trends: Increasing emphasis on personalized medicine, newborn genetic screening, and orphan drug incentives continues to support long-term relevance.
- Growing payer acceptance of ultra-orphan therapies strengthens reimbursement stability despite high pricing.
Golodirsen Recent Developments
- Sarepta has continued to report long-term follow-up data from ongoing extension studies of Golodirsen, demonstrating sustained dystrophin expression and stable functional outcomes over multiple years. The company has also emphasized Golodirsen’s role within its broader DMD treatment ecosystem, particularly as part of combination strategies alongside gene therapies and next-generation RNA platforms. In recent corporate updates, Sarepta reiterated its commitment to maintaining and optimizing its exon-skipping portfolio, positioning Golodirsen as a key revenue-stabilizing asset amid regulatory and commercial volatility surrounding newer gene therapy programs such as Elevidys.
“Golodirsen Sales Forecast, and Market Size Analysis – 2034” report provides comprehensive insights of Golodirsen for approved indication like Duchenne muscular dystrophy in the 7MM. A detailed picture of Golodirsen’s existing usage in anticipated entry and performance in approved indications in the 7MM, i.e., the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan for the study period 2020 –2034 is provided in this report along with a detailed description of the Golodirsen for approved indications. The Golodirsen market report provides insights about Golodirsen’s sales forecast, mechanism of action (MoA), dosage and administration, as well as research and development including regulatory milestones, along with other developmental activities. Further, it also consists of historical and current Golodirsen performance, future market assessments inclusive of the Golodirsen market forecast analysis for approved indications in the 7MM, SWOT, analysts’ views, comprehensive overview of market competitors, and brief about other emerging therapies in respective indications. It also provides analysis of Golodirsen sales forecasts, along with factors driving its market.
Golodirsen Drug Summary
Golodirsen is a morpholino antisense oligomer (PMO) administered intravenously to treat Duchenne muscular dystrophy (DMD) in patients with a confirmed mutation of the DMD gene amenable to exon 53 skipping. Developed by Sarepta Therapeutics and marketed as Vyondys 53, it received accelerated FDA approval on December 2019, based on increased dystrophin production in skeletal muscle, targeting approximately 8% of DMD patients. Golodirsen binds to exon 53 of dystrophin pre-mRNA, inducing its skipping during processing to restore the reading frame and enable production of a truncated, functional dystrophin isoform akin to that in Becker muscular dystrophy, potentially slowing muscle degeneration progression. The report provides Golodirsen’s sales, growth barriers and drivers, post usage and approvals in multiple indications.
Scope of the Golodirsen Market Report
The report provides insights into:
- A comprehensive product overview including the Golodirsen MoA, description, dosage and administration, research and development activities in approved indication like Duchenne muscular dystrophy.
- Elaborated details on Golodirsen regulatory milestones and other development activities have been provided in Golodirsen market report.
- The report also highlights Golodirsen‘s cost estimates and regional variations, reported and estimated sales performance, research and development activities in approved indications across the United States, Europe, and Japan.
- The Golodirsen market report also covers the patents information, generic entry and impact on cost cut.
- The Golodirsen market report contains current and forecasted Golodirsen sales for approved indications till 2034.
- Comprehensive coverage of the late-stage emerging therapies for respective indications.
- The Golodirsen market report also features the SWOT analysis with analyst views for Golodirsen in approved indications.
Methodology
The Golodirsen market report is built using data and information sourced primarily from internal databases, primary and secondary research and in-house analysis by DelveInsight’s team of industry experts. Information and data from the secondary sources have been obtained from various printable and nonprintable sources like search engines, news websites, global regulatory authorities websites, trade journals, white papers, magazines, books, trade associations, industry associations, industry portals and access to available databases.
Golodirsen Analytical Perspective by DelveInsight
In-depth Golodirsen Market Assessment
This Golodirsen sales market forecast report provides a detailed market assessment of Golodirsen for approved indication like Duchenne muscular dystrophy in the seven major markets, i.e., the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan. This segment of the report provides current and forecasted Golodirsen sales data uptil 2034.
Golodirsen Clinical Assessment
The Golodirsen market report provides the clinical trials information of Golodirsen for approved indications covering trial interventions, trial conditions, trial status, start and completion dates.
Golodirsen Competitive Landscape
The report provides Insights on competitors and marketed products within the domain, along with a summary of emerging products and their respective launch dates, posing significant competition in the market.
Golodirsen Market Potential & Revenue Forecast
- Projected market size for the Golodirsen and its key indications
- Estimated Golodirsen sales potential (Golodirsen peak sales forecasts)
- Golodirsen Pricing strategies and reimbursement landscape
Golodirsen Competitive Intelligence
- Number of competing drugs in development (pipeline analysis)
- Golodirsen Market positioning compared to existing treatments
- Golodirsen Strengths & weaknesses relative to competitors
Golodirsen Regulatory & Commercial Milestones
- Golodirsen Key regulatory approvals & expected launch timelines
- Commercial partnerships, licensing deals, and M&A activity
Golodirsen Clinical Differentiation
- Golodirsen Efficacy & safety advantages over existing drugs
- Golodirsen Unique selling points
Golodirsen Market Report Highlights
- In the coming years, the Golodirsen market scenario is set to change due to strong adoption, increased prescriptions and broader uptake in multiple immunological indications; which would expand the size of the market.
- The Golodirsen companies are developing therapies that focus on novel approaches to treat/improve the disease condition, assess challenges, and seek opportunities that could influence Golodirsen’s dominance.
- Other emerging products for Duchenne muscular dystrophy are expected to give tough market competition to Golodirsen and launch of late-stage emerging therapies in the near future will significantly impact the market.
- A detailed description of regulatory milestones, and developmental activities, provide the current development scenario of Golodirsen in approved indications.
- Analyse Golodirsen cost, pricing trends and market positioning to support strategic decision-making in the immunology landscape.
- Our in-depth analysis of the forecasted Golodirsen sales data uptil 2034 will support the clients in decision-making process regarding their therapeutic portfolio by identifying the overall scenario of Golodirsen in approved indications.
Key Questions
- What is the class of therapy, route of administration and mechanism of action of Golodirsen? How strong is Golodirsen’s clinical and commercial performance?
- What is Golodirsen’s clinical trial status in each individual indications such as Duchenne muscular dystrophy and study completion date?
- What are the key collaborations, mergers and acquisitions, licensing and other activities related to the Golodirsen Manufacturers?
- What are the key designations that have been granted to Golodirsen for approved indications? How are they going to impact Golodirsen’s penetration in various geographies?
- What is the current and forecasted Golodirsen market scenario for approved indications? What are the key assumptions behind the forecast?
- What are the current and forecasted sales of Golodirsen in the seven major countries, including the United States, Europe (Germany, France, Italy, Spain) and the United Kingdom, and Japan?
- What are the other emerging products available and how are these giving competition to Golodirsen for approved indications?
- Which are the late-stage emerging therapies under development for the treatment of approved indications?
- How cost-effective is Golodirsen? What is the duration of therapy and what are the geographical variations in cost per patient?