Granulomatosis with Polyangiitis Market
- Granulomatosis with polyangiitis, formerly known as Wegener granulomatosis, is a rare multisystem disease involving predominantly small vessels and is characterized by granulomatous inflammation, pauci-immune necrotizing glomerulonephritis, and vasculitis, leading to endothelial injury and tissue damage.
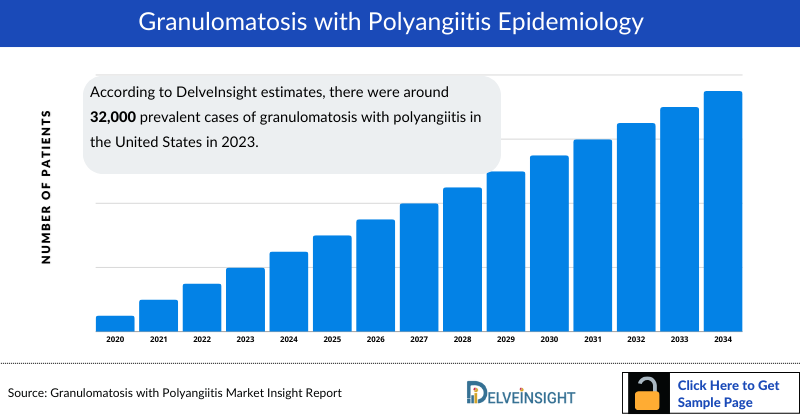
- According to DelveInsight estimates, there were around 32,000 prevalent cases of granulomatosis with polyangiitis in the United States in 2023. This number is expected to rise notably from 2020 to 2034. Contributing factors include advancements in diagnostic techniques, which are improving detection rates, an aging population that is more susceptible to autoimmune diseases, and increased awareness among healthcare providers leading to earlier and more accurate diagnoses.
- In 2023, about 50% of granulomatosis with polyangiitis (GPA) cases involved the upper and lower respiratory tracts. This is due to the disease's tendency to affect small-to-medium-sized blood vessels in these areas, leading to inflammation and granuloma formation, often resulting in chronic sinusitis, nasal obstruction, and lung nodules.
- In 2023, in the United States, a significant proportion of diagnosed prevalent cases received treatment. However, approximately 40% of these cases were classified as relapsed or refractory.
- The US FDA has approved two drugs for the treatment of granulomatosis with polyangiitis: TAVNEOS (avacopan) and RITUXAN (rituximab). TAVNEOS is developed by Chemocentryx/Amgen, while RITUXAN is developed by Genentech/Biogen.
- The pipeline for new treatments remains limited, underscoring a significant unmet need in managing this condition.
DelveInsight's “Granulomatosis with polyangiitis Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of the indication granulomatosis with polyangiitis, historical and forecasted epidemiology as well as the granulomatosis with polyangiitis market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The granulomatosis with polyangiitis market report provides real-world prescription pattern analysis, approved drugs, market share of individual therapies, and historical and forecasted 7MM granulomatosis with polyangiitis market size from 2020 to 2034. The report also covers current granulomatosis with polyangiitis treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Granulomatosis with polyangiitis Epidemiology |
Segmented by: · Total Prevalent Cases of Granulomatosis with polyangiitis in the 7MM [2020-2034] · Diagnosed Prevalent Cases of Granulomatosis with polyangiitis by Organ Involvement in the 7MM [2020-2034] · Diagnosed Prevalent Cases of Granulomatosis with polyangiitis by Antibody Type in the 7MM [2020-2034] · Diagnosed Prevalent Cases of Granulomatosis with polyangiitis by Severity in the 7MM [2020-2034] · Total Treated Cases of Granulomatosis with polyangiitis in the 7MM [2020-2034] |
|
Granulomatosis with polyangiitis Key Companies |
· Biogen/ Genentech · ChemoCentryx/ Amgen |
|
Granulomatosis with polyangiitis Key Therapies/Drug |
· TAVNEOS (avacopan) · RITUXAN (rituximab) |
|
Granulomatosis with polyangiitis Market |
Segmented By: · Region · Therapies |
|
Analysis |
· KOL Views · SWOT Analysis · Reimbursement · Conjoint Analysis · Unmet needs |
Granulomatosis with polyangiitis Treatment Market
Granulomatosis with polyangiitis Overview, Country-Specific Treatment Guidelines and Diagnosis
Granulomatosis with polyangiitis (GPA), formerly known as Wegener's granulomatosis, is a rare autoimmune disease characterized by inflammation of small to medium-sized blood vessels (vasculitis). This inflammation can lead to damage in various organs, particularly the respiratory tract and kidneys. It is classified as an ANCA-associated vasculitis (AAV) due to the presence of anti-neutrophil cytoplasmic antibodies (ANCAs) in most patients. The exact cause of granulomatosis with polyangiitis is unknown, but it likely involves an abnormal immune response triggered by environmental factors in genetically predisposed individuals. Clinical manifestations include chronic sinusitis, nasal ulcers, lung involvement (such as nodules or hemoptysis), and glomerulonephritis, which can lead to renal failure. Diagnosis is based on ANCA testing, tissue biopsy, and imaging studies.
The granulomatosis with polyangiitis report provides an overview of granulomatosis with polyangiitis pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis will be provided in the report...
Granulomatosis with Polyangiitis Treatment
The treatment approach for granulomatosis with polyangiitis (GPA) involves two main phases: induction and maintenance. Initially, induction therapy aims to rapidly control inflammation and halt disease progression, typically using a combination of rituximab or cyclophosphamide with glucocorticoids. For less severe cases, methotrexate may be used as an alternative. Once remission is achieved, the focus shifts to maintenance therapy to prevent relapse.
Azathioprine or methotrexate is commonly employed during this phase, often with lower doses of glucocorticoids. The treatment strategy is tailored to the individual’s disease severity, response to therapy, and potential side effects, ensuring long-term disease control with minimized toxicity.
Further details related to current treatment approach will be provided in the report...
Granulomatosis with Polyangiitis Epidemiology
The granulomatosis with polyangiitis epidemiology chapter in the report provides historical as well as forecasted prevalence in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The granulomatosis with polyangiitis epidemiology is segmented with detailed insights into- Total Prevalent Cases of Granulomatosis with polyangiitis, Diagnosed Prevalent Cases of Granulomatosis with polyangiitis by Organ Involvement, Diagnosed Prevalent Cases of Granulomatosis with polyangiitis by Antibody Type, Diagnosed Prevalent Cases of Granulomatosis with polyangiitis by Severity, Total Treated Cases of Granulomatosis with polyangiitis in the 7MM [2020-2034].
- The prevalence of granulomatosis with polyangiitis (GPA) in the United States, estimated at 32,000 cases in 2023, is expected to rise by 2034 due to factors like an aging population, improved diagnostics, an increase in autoimmune conditions, and environmental triggers.
- In 2023, about 50% of granulomatosis with polyangiitis (GPA) cases involved the upper and lower respiratory tracts. This is due to the disease's tendency to affect small-to-medium-sized blood vessels in these areas, leading to inflammation and granuloma formation, often resulting in chronic sinusitis, nasal obstruction, and lung nodules.
- In 2023, in the United States, a significant proportion of diagnosed prevalent cases received treatment. However, approximately 40% of these cases were classified as relapsed or refractory.
- EU4 and the UK, accounted for 55% of the total diagnosed prevalent cases of granulomatosis with polyangiitis in the year 2023.
Granulomatosis with polyangiitis Drug Chapters
The drug chapter segment of the granulomatosis with polyangiitis report encloses a detailed analysis of granulomatosis with polyangiitis marketed drugs. It also deep dives into the granulomatosis with polyangiitis pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Granulomatosis with polyangiitis Marketed Drugs
TAVNEOS (avacopan): Chemocentryx/Amgen
TAVNEOS (avacopan), approved by the FDA as an adjunctive treatment for adults with severe active ANCA-associated vasculitis, is a first-in-class, orally administered small molecule that employs a novel, highly targeted mode of action in complement-driven autoimmune and inflammatory diseases.
In October 2021, ChemoCentryx announced that the FDA had approved TAVNEOS (avacopan) as an adjunctive treatment of adult patients with severe active AAV, specifically GPA and MPA (the two main forms of AAV), in combination with standard therapy.
RITUXAN (rituximab): Genentech/Biogen
Rituximab is a monoclonal antibody that targets the CD20 antigen expressed on the surface of pre-B and mature B-lymphocytes. Upon binding to CD20, rituximab mediates B-cell lysis. Possible mechanisms of cell lysis include complement dependent cytotoxicity (CDC) and antibody dependent cell mediated cytotoxicity (ADCC). B cells are believed to play a role in the pathogenesis of rheumatoid arthritis (RA) and associated chronic synovitis.
In September 2019, the US Food and Drug Administration approved RITUXAN (rituximab) injection to treat granulomatosis with polyangiitis (GPA).
|
Approved Drugs | ||||||
|
Drug Name |
Company |
Indications |
Molecule Type |
Approval date |
MoA |
Designations |
|
TAVNEOS (avacopan) |
Chemocentryx/Amgen |
Granulomatosis with polyangiitis |
Small Molecule |
US: 2021 |
C5a receptor antagonist |
NA |
|
RITUXAN (rituximab) |
Genentech/Biogen |
Granulomatosis with polyangiitis |
Small Molecule |
US: 2019 |
Anti-CD20 monoclonal antibody |
NA |
Note: Detailed current therapies assessment will be provided in the full report of granulomatosis with polyangiitis...
Granulomatosis with polyangiitis Market Outlook
The market size for granulomatosis with polyangiitis in the seven major markets is projected to experience significant growth from 2024, driven by a notable increase in prevalent cases and the necessity for innovative therapies that can provide more effective, long-term relief for the thousands of patients affected by granulomatosis with polyangiitis.
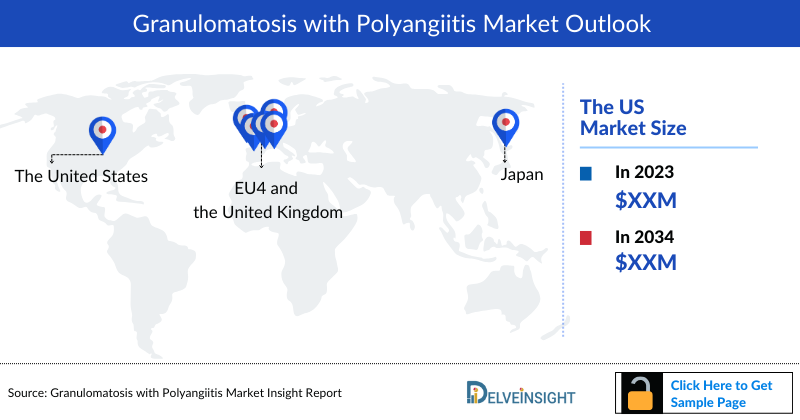
- The United States accounts for the largest market size of granulomatosis with polyangiitis, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Among EU4 and the UK, Germany had the largest market size contributing about, followed by UK, with Spain having the smallest market size in 2023.
- In 2023, TAVNEOS led the granulomatosis with polyangiitis market, achieving a notable market value of USD 70 million. Glucocorticosteroids and immunosuppressive therapies also held substantial market shares.
Granulomatosis with polyangiitis Drug Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Granulomatosis with polyangiitis Activities
This section provides insights into different Granulomatosis with polyangiitis clinical trials. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
This section covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies.
KOL Views
To keep up with the real-world scenario in current market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current treatment patterns of granulomatosis with polyangiitis. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy.
Market Access and Reimbursement
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Granulomatosis with polyangiitis Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of granulomatosis with polyangiitis, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of the current therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the granulomatosis with polyangiitis market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM granulomatosis with polyangiitis.
Granulomatosis with polyangiitis Report Insights
- Granulomatosis with polyangiitis Patient Population
- Granulomatosis with polyangiitis Therapeutic Approaches
- granulomatosis with polyangiitis Pipeline Analysis
- granulomatosis with polyangiitis Market Size and Trends
- Existing and future Market Opportunity
Granulomatosis with polyangiitis Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- granulomatosis with polyangiitis Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved therapies
- Key Cross Competition
- Conjoint analysis
- Granulomatosis with polyangiitis Drugs Uptake
- Key Granulomatosis with polyangiitis Market Forecast Assumptions
Granulomatosis with polyangiitis Report Assessment
- Current Granulomatosis with polyangiitis Treatment Practices
- Granulomatosis with polyangiitis Unmet Needs
- Granulomatosis with polyangiitis Pipeline Product Profiles
- Granulomatosis with polyangiitis Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Granulomatosis with polyangiitis Market Drivers
- Granulomatosis with polyangiitis Marekt Barriers
FAQs
- What is the growth rate of the 7MM granulomatosis with polyangiitis treatment market?
- What was the granulomatosis with polyangiitis total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current options for the treatment of granulomatosis with polyangiitis?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy Granulomatosis with polyangiitis Market Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the granulomatosis with polyangiitis Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Detailed analysis and ranking of class-wise potential current therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.