Hairy Cell Leukemia Market
- The Hairy Cell Leukemia Market Size is anticipates to grow with a significant CAGR during the study period (2020-2034).
- While hairy cell leukemia cannot be cured, great progress in the treatment of hairy cell leukemia has resulted in prolonged survival for many patients. Most patients respond well to treatment with purine analogues. Purine nucleoside analogs remain the cornerstone of treatment, and the addition of rituximab has deepened and prolonged responses in the upfront and relapsed setting.
- Hairy cell leukemia is a rare B cell neoplasm that accounts for 2% of B-cell lymphomas. Most HCL cases are postulated to be derived from BRAF V600E gene mutation of late activated memory B cells.
- KMT2C mutations occur in 15% and 25% of patients with classical hairy cell leukemia (cHCL) and variant hairy cell leukemia (vHCL), respectively, along with CCND3 and U2AF1 mutations each in 13% of vHCLs. NF1, NF2, N/KRAS, and IRS1 alterations contribute to clinical resistance to vemurafenib treatment in patients with cHCL.
- In November 2022, AstraZeneca announced to permanently discontinue LUMOXITI (moxetumomab pasudotox-tdfk) from the US market by July 2023 and advised distributors to stop all distribution in August 2023. The withdrawal was related to its very low clinical uptake due to the availability of other treatment options. Complex administration and the need for toxicity prophylaxis and safety monitoring may also have contributed to its low uptake. The removal is not related to the safety or efficacy of the drug.
- The future of HCL treatment is likely to focus on personalized approaches, with therapies tailored to specific genetic mutations, such as BRAF and CD19.
- BRAF inhibitors (vemurafenib) have transformed the treatment of HCL by greatly enhancing patient outcomes while reducing side effects. As a result, these treatments are becoming the preferred choice for patients and healthcare providers. Pharmaceutical companies are also heavily investing in the research and development of new targeted therapies, solidifying BRAF inhibitors' position as safer and more effective alternatives to traditional chemotherapy in the HCL market. As of now vemurafenib is not approved for HCL.
- The leading Hairy Cell Leukemia companies such as Roche, Innate Pharma, Johnson & Johnson, Amega Biotech, LAVA Therapeutics, and others are dveloping therapies for Hairy Cell Leukemia treatment.
Download the Sample PDF to Get More Insight @ Hairy Cell Leukemia Market Forecast
DelveInsight's “Hairy Cell Leukemia Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of historical and forecasted epidemiology as well as the hairy cell leukemia therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Hairy cell leukemia market report provides current treatment, emerging therapies, market share of individual therapies, and historical and forecasted 7MM hairy cell leukemia market size from 2020 to 2034. The report also covers current hairy cell leukemia treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Hairy Cell Leukemia Market |
|
|
Hairy Cell Leukemias Market Size | |
|
Hairy Cell Leukemia Companies |
Roche, Innate Pharma, Johnson & Johnson, Amega Biotech, LAVA Therapeutics, and others |
|
Hairy Cell Leukemia Epidemiology Segmentation |
|
Hairy Cell Leukemia Treatment Market
Hairy Cell Leukemia Overview
Hairy cell leukemia is a rare type of blood cancer characterized by abnormal changes in white blood cells known as B lymphocytes. The bone marrow creates too many of these defective cells, known as “hairy cells” because of the thin hair-like projections found on their surface. Overproduction and accumulation of hairy cells causes a deficiency of normal blood cells (pancytopenia), including an abnormal decrease of certain white blood cells (neutrophils [neutropenia]) and certain red blood cells (platelets [thrombocytopenia]). Affected individuals usually exhibit fatigue, weakness, fever, weight loss, and/or abdominal discomfort due to an abnormally enlarged spleen (splenomegaly). In addition, affected individuals may have a slightly enlarged liver (hepatomegaly) and may be unusually susceptible to bruising and/or severe infection.
Hairy Cell Leukemia Diagnosis
Diagnosis of hairy cell leukemia is based on morphological evidence of hairy cells under microscopic examination. The hairy cell leukemia cell is a mononuclear cell that is usually one to two times the size of a mature lymphocyte. Hairy cell leukemia cells can be identified in Romanowsky-stained peripheral blood films from approximately 90% of patients as mononuclear cells that are usually one to two times the size of a mature lymphocyte.
The best approach to establishing the diagnosis of hairy cell leukemia is to carefully examine blood and bone marrow biopsy specimens to identify cells with the morphologic features of hairy cells and to demonstrate that the neoplastic cells have an antigenic profile that is characteristic for hairy cell leukemia. In addition to a physical exam, following tests and procedures can be performed such as complete blood count (CBC), peripheral blood smear, flow cytometry, bone marrow aspiration and biopsy, immunophenotyping, BRAF gene testing, and CT scan.
Further details related to country-based variations in diagnosis are provided in the report...
Hairy Cell Leukemia Treatment
Hairy cell leukemia is usually slow growing, and not all newly diagnosed patients with hairy cell leukemia require immediate treatment. For approximately 10% of patients, if they have stable blood counts and no symptoms at the time of diagnosis, the treatment may be the “watch-and-wait” approach. Watch and-wait is an appropriate medical approach that means treatment is delayed until signs and symptoms of the disease appear or progress. Some patients with hairy cell leukemia live for many years without any symptoms and without receiving any treatment. Frequent monitoring, including blood testing, is necessary so that treatment can be started if the disease begins to advance. Highly effective treatments are available for eligible patients. Alpha interferon (Intron-A and Roferon-A) was approved for hairy cell leukemia in 1986. NIPENT (pentostatin) was approved as a second- line therapy in 1991. Later in 1993, FDA approved LEUSTATIN (cladribine) for the treatment of hairy cell leukemia. The backbone of therapy remains a purine nucleoside analog either in the form of cladribine or pentostatin which have traditionally been used as monotherapies. Rituximab may also be added to cladribine although these drugs have not been directly compared in randomized trials, they appear to be equally effective. On the other hand, some patients do not respond to treatment at all, and still others respond at first, but over time their disease relapses and they require additional treatment. Ongoing Hairy cell leukemia clinical trials are exploring novel therapies and combination treatments aimed at improving remission rates, reducing relapse, and enhancing quality of life for affected patients.
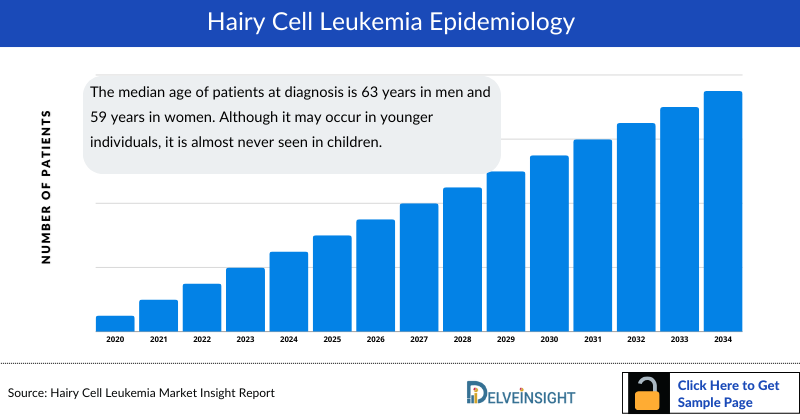
Hairy Cell Leukemia Epidemiology
The hairy cell leukemia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total incident cases of hairy cell leukemia, incident cases of hairy cell leukemia by symptoms, age-specific cases hairy cell leukemia, gender-specific cases of hairy cell leukemia, and line-wise treatment eligible cases of hairy cell leukemia in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- The incidence of hairy cell leukemia is very low in Asia, including Japan, and Africa, but is relatively high in Europe and the United States, where it accounts for 2-3% of all leukemia cases, with 3.5 cases per million per year.
- Hairy cell leukemia is very rare, around 230 people are diagnosed with hairy cell leukemia each year in the United Kingdom.
- Hairy cell leukemia is four to five times more common in men than women.
- The median age of patients at diagnosis is 63 years in men and 59 years in women. Although it may occur in younger individuals, it is almost never seen in children.
Hairy Cell Leukemia Drug Chapters
The hairy cell leukemia drug chapter segment encloses a detailed analysis of hairy cell leukemia early and mid-stage (Phase I, and Phase II) hairy cell leukemia pipeline drugs. It also deep dives into the hairy cell leukemia pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations. The Hairy Cell Leukemia drugs market is witnessing steady growth due to increased research initiatives, improved diagnostics, and the development of targeted therapies aimed at enhancing patient outcomes and survival.
Hairy Cell Leukemia Emerging Drugs
TECARTUS (brexucabtagene autoleucel): Gilead Sciences
TECARTUS is a CD19-directed genetically modified autologous T-cell immunotherapy that binds to CD19-expressing cancer cells and normal B-cells. It was already approved in mantle cell lymphoma and acute lymphoblastic leukemia.
Now the company is conducting Phase II trial for relapsed/refractory hairy cell leukemia. The goal of these trials is to see if CARs can help T cells identify and attack HCL cells, and if increasing the doses of CAR T cells is safe.
MGD024: MacroGenics
MGD024 is an investigational, second-generation CD123 × CD3 DART molecule that can simultaneously bind CD123 on malignant cells and CD3 on T cells, targeting CD123+ leukemic cells for recognition and elimination by CD3+ T lymphocytes as effector cells. In October 2022, MacroGenics announced that it had entered into an exclusive option and collaboration agreement with Gilead to develop MGD024 and two additional bispecific research programs. MacroGenics is responsible for the ongoing Phase I study for MGD024 during which Gilead may elect to exercise its option to license the program at predefined decision points.
LP-168: Newave Pharmaceutical
LP-168 is a selective next generation inhibitor of BTK that can bind wild-type and C481-mutated BTK covalently and non-covalently, respectively, with preclinical activity in resistant CLL models. This dual activity is hypothesized to allow efficacy against cells with wild-type BTK through covalent activity while preventing expansion of the most common resistance mechanisms through non-covalent activity. Currently Newave Pharmaceutical is running a Phase I, multi-center, open-label, dose-escalation study to evaluate the safety, tolerability, pharmacokinetics and clinical activity of LP-168 in subjects with relapsed or refractory B-cell malignancies including hairy cell leukemia.
|
Comparison of key emerging drugs | ||||||
|
Drug name |
Company |
MoA |
RoA |
Molecule type |
Phase |
Indication |
|
Brexucabtagene autoleucel |
Gilead Sciences |
Targeting CD19 |
IV |
CAR-T cell therapy |
II |
Relapsed/Refractory Hairy Cell Leukemia |
|
ACP-196 (acalabrutinib) |
Acerta Pharma |
BTK inhibitor |
Oral |
Small molecule |
I/II |
Hairy Cell Leukemia |
|
MGD024 |
MacroGenics |
CD123 × CD3 |
IV |
Bispecific molecule |
I |
Hairy Cell Leukemia |
|
LP-168 |
Newave Pharmaceutical |
BTK inhibitor |
Oral |
Small molecule |
I |
Hairy Cell Leukemia |
Note: Detailed emerging therapies assessment will be provided in the final report...
Hairy Cell Leukemia Drug Class Insights
Systemic therapy of hairy cell leukemia has changed rapidly in the past 10 years because of new biologic agents (eg, interferons) and new purine analogues. Also, the availability of recombinant human hematopoietic growth factors has improved supportive care during life-threatening infections in these patients. Currently CD-19 CAR-T cell therapy and BTK inhibitors are being explored for the treatment of hairy cell leukemia.
Purine Analogues: Purine analogues are a standard treatment for hairy cell leukemia. These drugs include cladribine and pentostatin. Both drugs are equally effective at inducing and maintaining remission. In most patients, purine analogues can induce long-term remission. Purine analogues have been one of the biggest successes in cancer treatment history. Before the early 1980s, the median overall survival for HCL patients was around four years. However, patients who are diagnosed at a median age of around 55 years still have a significant risk of relapse later in life. Has a 94% overall response and 84% complete response in hairy cell leukemia. Long term use causes serious side effects and relapse.
Hairy Cell Leukemia Market Outlook
Advances in the understanding of hairy cell leukemia have led to new approaches in management and improved outcomes; nonetheless, there is room for improvement. Over the past decade, there has been enormous progress in the understanding of the biology of hairy cell leukemia which has led to the development of novel therapeutic strategies. The maturation of data regarding existing management strategies has also lent considerable insight into therapeutic outcomes and prognosis of patients treated with chemo- or chemoimmunotherapy. Purine nucleoside analogs remain the cornerstone of treatment, and the addition of rituximab has deepened and prolonged responses in the upfront and relapsed setting. Targeted therapies now have a more defined role in the management of hairy cell leukemia, with BRAF inhibitors now having a potential in the first-line setting in selected cases as well as in relapse. Next-generation sequencing for the identification of targetable mutations, evaluation of measurable residual disease, and risk stratification continue to be areas of active investigation
While hairy cell leukemia cannot be cured with currently available therapies, highly effective treatments are available for eligible patients. The backbone of therapy remains a purine nucleoside analog (PA), either in the form of cladribine or pentostatin which have traditionally been used as monotherapies. Although these drugs have not been directly compared in randomized trials, they appear to be equally effective for the achievement of CR and PR (76–83% and 31–33%, respectively). Risk stratification continues to be an area of active investigation, and additional studies of molecular and genetic predictors of outcomes will further refine future treatment recommendations. Hairy cell leukemia remains a success story highlighting the positive impact of large multicenter clinical trials in rare diseases which in this case changed a universally fatal illness into one with a survival comparable to that of unaffected counterparts.
Hairy Cell Leukemia Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, efficacy data, and order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake. CD19 CAR-T therapy TECARTUS is being investigated for the treatment of hairy cell leukemia. Since no other drug besides purine analogs is currently approved, if TECARTUS demonstrates strong efficacy and safety, it could capture a significant share of the patient population, leading to rapid market adoption.
Further detailed analysis of emerging therapies drug uptake in the report...
Hairy Cell Leukemia Activities
The Hairy Cell Leukemia pipeline report provides insights into different Hairy Cell Leukemia clinical trials within Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Hairy Cell Leukemia Pipeline Development Activities
The Hairy Cell Leukemia clinical trials analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for hairy cell leukemia emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Ophthalmologists, Professors, and others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 7+ KOLs in the 7MM. Centers such as the Center for Integrated Oncology (CIO), John Mendelsohn Faculty Center, Sidney Kimmel Medical College, the School of the Johns Hopkins University Wilmer Eye Institute, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or hairy cell leukemia market trends.
|
Region |
KOL Views |
|
Germany |
“Despite the fact that more effective chemotherapeutics are available in the treatment of HCL today, splenectomy still remains a valid treatment option in certain cases. “ |
|
United States |
“While dramatic improvements in the treatment of hairy cell leukemia have occurred in the past three decades and outcomes are generally excellent, the disease remains incurable and sequential therapies are associated with cumulative toxicity. “ |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most crucial primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and this clearly explains the drug's side effects in the trials. In addition, the scoring is also based on the probability of success and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Hairy Cell Leukemia Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of hairy cell leukemia, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current treatment landscape.
- A detailed review of the hairy cell leukemia market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM hairy cell leukemia Market.
Hairy Cell Leukemia Market Report Insights
- Hairy Cell Leukemia Patient Population
- Hairy Cell Leukemia Therapeutic Approaches
- Hairy Cell Leukemia Pipeline Analysis
- Hairy Cell Leukemia Market Size and Trends
- Existing and future Market Opportunity
Hairy Cell Leukemia Market Report Key Strengths
- Eleven Years Forecast
- The 7MM Coverage
- Hairy Cell Leukemia Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Hairy Cell Leukemia Drugs Uptake
- Key Hairy Cell Leukemia Market Forecast Assumptions
Hairy Cell Leukemia Market Report Assessment
- Current Hairy Cell Leukemia Treatment Practices
- Hairy Cell Leukemia Unmet Needs
- Hairy Cell Leukemia Pipeline Product Profiles
- Hairy Cell Leukemia Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Hairy Cell Leukemia Market Drivers
- Hairy Cell Leukemia Market Barriers
FAQs
- What is the historical and forecasted hairy cell leukemia patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- What was the hairy cell leukemia total market size, the market size by therapies, market share (%) distribution in 2023, and what would it look like in 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for treating hairy cell leukemia?
- How many companies are developing therapies to treat hairy cell leukemia?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buyHairy Cell Leukemia Market Forecast Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the hairy cell leukemia Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.