Hepatic Encephalopathy Market
- The Hepatic Encephalopathy Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Hepatic Encephalopathy market size from 2020 to 2034. The report also covers current Hepatic Encephalopathy treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the market.
- Hepatic Encephalopathy Diagnosed Prevalence is expected to rise, driven by increased awareness, increased health care concerns, longer life expectancy as well as changes in cirrhosis admission.
- In 2023, the US accounted for the maximum diagnosed patient share of Hepatic Encephalopathy in the 7MM, i.e., 61%, followed by Japan accounting for around 9% of the total 7MM cases. Among the European countries higher number of cases were in the UK accounting for about 8% of the total 7MM cases.
- In 2023, the market size of Hepatic Encephalopathy was highest in the US among the 7MM accounting for approximately USD 1460 million that is further expected to increase by 2034.
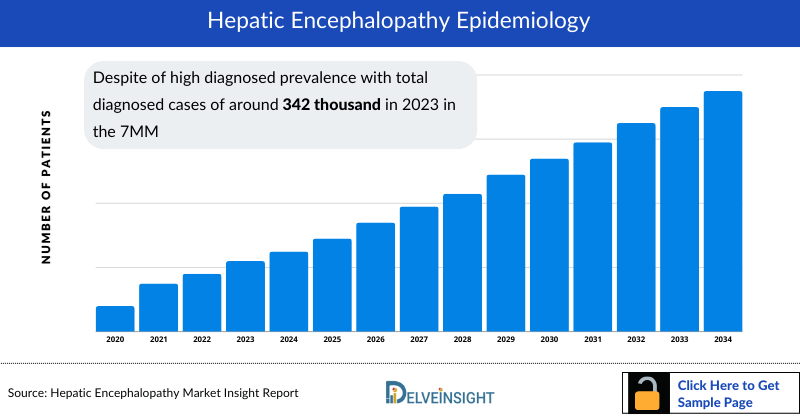
- Despite of high diagnosed prevalence with total diagnosed cases of around 342 thousand in 2023 in the 7MM, the treatment market of Hepatic Encephalopathy has very less therapies specific to Hepatic Encephalopathy treatment.
- Emerging therapies have the potential to create a significant positive shift in the Hepatic Encephalopathy Treatment Market Size.
DelveInsight's “Hepatic Encephalopathy Market Insights, Epidemiology and Market Forecast– 2034” report delivers an in-depth understanding of the Hepatic Encephalopathy, historical and forecasted epidemiology as well as the Hepatic Encephalopathy market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Request a sample to unlock the CAGR for the Hepatic Encephalopathy Treatment Market
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024 to 2034 |
|
Geographies Covered |
|
|
Hepatic Encephalopathy Treatment Market |
|
|
Hepatic Encephalopathy Market Size | |
|
Hepatic Encephalopathy Companies |
|
Hepatic Encephalopathy Treatment Market
Hepatic encephalopathy is a brain disorder that develops in some individuals with liver disease. It is a complex disorder encompassing a spectrum or continuum of disease that ranges from a subtle condition with no outward signs or symptoms to a severe form that can cause serious, life-threatening complications. hepatic encephalopathy is classified into two broad categories based on severity, covert hepatic encephalopathy (CHE) and overt hepatic encephalopathy (OHE). Presently, defining and diagnosing hepatic encephalopathy is quite challenging. In CHE, there are no clear clinical signs or symptoms as presented in OHE.
It is presumed that hepatic encephalopathy is a predictor of death. The hepatic functions and prognosis get worse after the diagnosis of the disease. The patients are often referred to a liver transplant center at a later stage, which is not a very economical option. Also, this disease levies a significant burden on patients, such as psychological and social functioning, other than morbidity and mortality. Expenditures related to patients’ caregivers, hospitalization, and society get increased. Moreover, hepatic encephalopathy can impact the patient’s ability to work, resulting in reduced productivity and lost wages. Thus, the overall quality of life of patients gets hampered. Given the social and financial burden of HE, cost-effective management of hepatic encephalopathy is crucial. Early prevention is important to minimize the societal and economic costs associated with hepatic encephalopathy.
Hepatic Encephalopathy Diagnosis
Hepatic encephalopathy is a common complication of liver dysfunction, including acute liver failure and liver cirrhosis. hepatic encephalopathy presents as a spectrum of neuropsychiatric symptoms ranging from subtle fluctuating cognitive impairment to coma. There is no specific diagnostic test for HE, and diagnosis is based on clinical suspicion, excluding other causes and the use of clinical tests that may support its diagnosis. Many tests are used in trials but have not yet gained universal acceptance. Currently, diagnosis is based on eliminating other causes, establishing liver disease, and psychometric testing. Ammonia (regardless of level) does not add any diagnostic, staging, or prognostic value for hepatic encephalopathy. Also, there is a long list of disorders that could mimic or associate with hepatic encephalopathy and are considered for purposes of differential diagnosis. Thus, there is an unmet need to develop diagnostic tests (laboratory or imaging) which help early diagnosis and treatment monitoring.
None of the manifestations of type CHE are specific, and there are no clinical markers, which are truly useful in distinguishing between OHE and other neurological alterations of metabolic origin that may occur in patients with cirrhosis but are not causally related to liver disease. Ammonia levels within the normal range have a high negative predictive value and virtually no false negatives on measurement. Thus, a prerequisite of diagnostic guidelines and specific procedures for diagnosing hepatic encephalopathy and its subtypes are current unmet needs.
Further details related to country-based variations are provided in the report...
Hepatic Encephalopathy Treatment
Current treatment of hepatic encephalopathy and its prevention relies on lactulose and rifaximin. Both the therapies have limited use due to low compliance because both the therapies have associated side effects. Lactulose possesses key side effects such as difficulty administering in acute settings, and poor compliance due to GI effects. Rifaximin contains side effects such as peripheral edema, GI issues, nausea, and dizziness. Approximately 55—65% of high-risk patients receive current SOC, and very few remain on therapy due to poor compliance. Also, rifaximin is not indicated for severe hepatic impairment as systemic exposure is higher in those patients; thus when administering Rifaximin to patients with severe hepatic impairment (Child-Pugh C), caution is exercised. The clinical trials were also limited to patients with MELD scores < 25. These have an indirect effect on ammonia levels and, therefore, unsuccessful in several patients. Drugs with novel and targeted MoA with proven clinical efficacy are required to manage episodes of OHE and improve neurocognition. Improvement in West Harvey Score generally takes more than 24 h after initiation of lactulose therapy. Better and quicker management of OHE episodes is required to reduce hospital stays and associated costs. Due to indirect MOA, the response usually comes after 24 h. The current pipeline is also not very robust for the treatment of HE. Novel therapies for hospital or outpatient settings and to prevent recurrence are key unmet needs.
Hepatic Encephalopathy Epidemiology
As the market is derived using the patient-based model, the Hepatic Encephalopathy epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Hepatic Encephalopathy Diagnosed Prevalent Cases, Hepatic Encephalopathy Gender-specific Diagnosed Prevalent Cases, Hepatic Encephalopathy Age-specific Diagnosed Prevalent Cases, and Hepatic Encephalopathy Type-specific Diagnosed Prevalent Cases in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- The total Hepatic Encephalopathy diagnosed prevalent cases in the US are expected to increase with a significant CAGR by 2034, from around 209 thousand cases in 2023 in the US.
- Among the European countries, the UK (28%) had the highest diagnosed prevalent population of hepatic encephalopathy, followed by Germany in 2023. On the other hand, Spain had the least diagnosed prevalent population around 9% of Hepatic Encephalopathy in the same year.
- In Japan, among diagnosed prevalence cases of Hepatic Encephalopathy, most cases were of Covert Hepatic Encephalopathy (~17.7 thousand) in 2023. While Overt Hepatic Encephalopathy cases were around 11.8 thousand in the same year.
- The Hepatic Encephalopathy diagnosed cases were segmented based on age in seven age‐groups <25 years, 25-34 years, 35-44 years, 45-54 years, 55-64 years, 65-74 years, and 75+ years, Our estimate suggests that in the US highest number of cases were in the age goup 45-54 years (41%) and the least in <25 years (3%) in 2023.
- Assessments as per DelveInsight’s analysts show that the majority of cases of Hepatic Encephalopathy are occupied by males as compared to females. There were approximately 152 thousand male and 57 thousand female cases of Hepatic Encephalopathy in 2023 in the US.
Hepatic Encephalopathy Drug Chapters
The drug chapter segment of the Hepatic Encephalopathy therapeutics market report encloses a detailed analysis of Hepatic Encephalopathy marketed drugs and late-stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Hepatic Encephalopathy clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Hepatic Encephalopathy news and press releases.
Hepatic Encephalopathy Marketed Drugs
- Xifaxan (Rifaximin/OHE7): Salix Pharmaceuticals/Bausch Health
Xifaxan (rifaximin) tablets (550 mg) are indicated for the reduction in risk of Overt Hepatic Encephalopathy recurrence in patients ≥ 18 years of age. Rifaximin has been granted Orphan Drug designation by the FDA for use in hepatic encephalopathy. In addition, guidelines from the AASLD and EASL recommend rifaximin as an add-on therapy to lactulose to reduce the risk of OHE recurrence.
Note: Detailed current therapies assessment will be provided in the full report of Hepatic Encephalopathy...
Hepatic Encephalopathy Emerging Drugs
- Golexanolone: Umecrine Cognition AB
Golexanolone, is a novel small molecule GABAA receptor-modulating steroid antagonist in development for the treatment of neurological disorders caused by overactivation of GABA-A receptors by the neurosteroid allopregnanolone. Golexanolone prevents the harmful effects induced by elevated levels of allopregnanolone in the brain. In contrast to traditional GABA-A antagonists, golexanolone can not completely block the receptor’s function and thereby induce side effects such as seizures and unconsciousness. Golexanolone is currently under development for the treatment of two disorders – Primary Biliary Cholangitis (PBC) and Hepatic Encephalopathy.
Note: Detailed emerging therapies assessment will be provided in the final report...
Hepatic Encephalopathy Drugs Market Insights
The current Hepatic Encephalopathy therapeutics market landscape is dependent on the use of medications. Common medications which are used in the management of HE in the United States include lactulose and rifaximin. Nutrition may also play a key role in managing HE and preventing a recurrence. The second line, less accepted therapies, include probiotics, branched-chain amino acids (BCAAs), flumazenil, zinc, and ammonia scavengers. Other agents that are used include oral antibiotics (neomycin, metronidazole, and rifaximin) that can reduce urease-producing bacteria in the intestines, resulting in a decrease in ammonia production and absorption through the gastrointestinal tract. Both neomycin and metronidazole have been used for many years to treat HE and are inexpensive.
Hepatic Encephalopathy Market Outlook
The current Hepatic Encephalopathy treatment involves reducing ammonia levels, which is the central therapeutic strategy. This can be achieved through various interventions, including dietary protein supplementation, purgatives such as nonabsorbable disaccharides and enemas, nonabsorbable antibiotics, modulation of interorgan ammonia using L-ornithine, L-aspartate (LOLA), sodium benzoate, and phenylacetate, and others like flumazenil, bromocriptine, acarbose, probiotics, and emerging therapies like LOLA, sodium benzoate, and acetyl L-carnitine.
In addition to these, rifaximin, an orally administered and non-absorbable antibiotic, is an alternative when lactulose is not tolerated or can be used concurrently. Lactulose and lactitol are also commonly used to treat HE. For severe HE requiring ventilation, early airway maintenance, sedation, and mechanical ventilation are essential to protect the airway and prevent high carbon dioxide tension and hypoxia. Once intubated, the head should be elevated by 10-20° with minimal intervention and care when moving patients to optimize intracranial pressure.
It is important to note that there is a need for newer novel therapies, particularly for patients with advanced HE and worsening acute liver injury, as reduction of plasma ammonia remains the central strategyThe recent advancement in therapeutic approaches and various novel targets open the market for new emerging therapies. New therapeutic approaches for Hepatic Encephalopathy under development include golexanolone, and others.
Umecrine Cognition is developing novel GR3027 (golexanolone), an orally administrated small molecule to treat patients diagnosed with HE. It belongs to a novel class of neurosteroid-based drugs for oral administration. Enhanced GABAA receptor signaling is implicated in key neurological symptoms associated with HE, such as impaired cognitive- and motor functions. Initial trial results have shown that it is effective in improving cognitive functions and might be a game-changer in the treatment of CHE, as no treatment is currently established for this subtype.
- The total Hepatic Encephalopathy Market Size in the 7MM was ~USD 1,680 Million in 2023 and is projected to increase during the forecast period (2024-2034).
- The Hepatic Encephalopathy Market Size in the 7MM will increase at a constant CAGR due to increasing awareness of the disease, better diagnosis, and the launch of the emerging target therapy for Hepatic Encephalopathy.
- Among EU4 and the UK, Germany with a share of 31% accounted for the maximum Hepatic Encephalopathy market size in 2023 while Spain occupied the bottom of the ladder with a share of 10% in 2023.
- In 2023, Japan held the second-largest Hepatic Encephalopathy market share, approximately 4%, of the hepatic encephalopathy treatment market among the seven major markets (7MM).
Hepatic Encephalopathy Drugs Uptake
This section focuses on the rate of uptake of the potential Hepatic Encephalopathy drugs expected to get launched in the market during the study period 2020-2034. For example, for Golexanolone (GR3027), we expect the drug uptake to be medium with a probability-adjusted peak share of around 45%, and years to the peak is expected to be 6 years from the year of launch in the US.
Hepatic Encephalopathy Pipeline Development Activities
The Hepatic Encephalopathy treatment market report provides insights into Hepatic Encephalopathy clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key Hepatic Encephalopathy Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Hepatic Encephalopathy treatment market report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for Hepatic Encephalopathy emerging therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Hepatic Encephalopathy Treatment Drugs
KOL- Views
To keep up with current Hepatic Encephalopathy Therapeutics Market Trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Hepatic Encephalopathy evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility, include NewYork-Presbyterian, and Weill Cornell Medicine, USA; University of California, Los Angeles, US; UCL Institute for Liver and Digestive Health, UK; P University of Padova, Italy; University of Washington, US, and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights, however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or Hepatic Encephalopathy market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and Hepatic Encephalopathy Therapeutics Market Intelligence analysis using various approaches, such as SWOT analysis, and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis is done to analyze multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Hepatic Encephalopathy Therapeutics Market Access and Reimbursement
Reimbursement is a crucial point for any drug after its approval. Many drugs or therapies are not properly recognized by the reimbursement body and may fail to get reimbursed or their reimbursement process gets delayed. The Hepatic Encephalopathy therapeutics market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of The Hepatic Encephalopathy Therapeutics Market Report
- The Hepatic Encephalopathy therapeutics market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression along with treatment guidelines
- Additionally, an all-inclusive account of both the current and emerging therapies along with the elaborative profiles of late-stage and prominent therapies will have an impact on the current Hepatic Encephalopathy treatment market landscape
- A detailed review of the Hepatic Encephalopathy therapeutics market; historical and forecasted Hepatic Encephalopathy market size, Hepatic Encephalopathy market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach
- The Hepatic Encephalopathy therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preference that help in shaping and driving the 7MM Hepatic Encephalopathy drugs market
Hepatic Encephalopathy Therapeutics Market Report Insights
- Patient-based Hepatic Encephalopathy Market Forecasting
- Hepatic Encephalopathy Therapeutic Approaches
- Hepatic Encephalopathy Pipeline Analysis
- Hepatic Encephalopathy Market Size and Trends
- Existing and future Hepatic Encephalopathy Drugs Market Opportunity
Hepatic Encephalopathy Therapeutics Market Report Key Strengths
- 11 Years Hepatic Encephalopathy Market Forecast
- 7MM Coverage
- Hepatic Encephalopathy Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Hepatic Encephalopathy Drugs Uptake
- Key Hepatic Encephalopathy Market Forecast Assumptions
Hepatic Encephalopathy Therapeutics Market Report Assessment
- Current Hepatic Encephalopathy Treatment Market Practices
- Hepatic Encephalopathy Unmet Needs
- Hepatic Encephalopathy Pipeline Product Profiles
- Hepatic Encephalopathy Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Hepatic Encephalopathy Market Drivers
- Hepatic Encephalopathy Market Bariers
Key Questions Answered In The Hepatic Encephalopathy Market Report:
Hepatic Encephalopathy Drugs Market Insights:
- What was the Hepatic Encephalopathy market size, the Hepatic Encephalopathy market size by therapies, and market share (%) distribution in 2020, and how it would all look in 2034? What are the contributing factors for this growth?
- What are the unmet needs are associated with the current Hepatic Encephalopathy treatment market?
- How is Golexanolone (GR3027) going to contribute to the Hepatic Encephalopathy Drugs Market after approval?
- Which drug is going to be the largest contributor in 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Hepatic Encephalopathy Epidemiology Insights:
- What are the disease risk, burden, and Hepatic Encephalopathy unmet needs? What will be the growth opportunities across the 7MM concerning the Hepatic Encephalopathy patient population?
- What is the historical and forecasted Hepatic Encephalopathy patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Why do only limited patients appear for diagnosis? Why is the current year diagnosis rate not high?
- What factors are affecting the diagnosis and treatment of the indication?
Current Hepatic Encephalopathy Treatment Market Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options for the Hepatic Encephalopathy treatment? What are the current treatment guidelines for the Hepatic Encephalopathy treatment in the US and Europe?
- How many companies are developing therapies for the Hepatic Encephalopathy treatment?
- How many emerging therapies are in the mid-stage and late stage of development for the Hepatic Encephalopathy treatment?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Hepatic Encephalopathy?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted Hepatic Encephalopathy Market?
Reasons to Buy Hepatic Encephalopathy Market Forecast Report
- The Hepatic Encephalopathy Drugs Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Hepatic Encephalopathy Therapeutics Market
- Insights on patient burden/disease Hepatic Encephalopathy Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- To understand the existing Hepatic Encephalopathy Drugs Market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Hepatic Encephalopathy Drugs Market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Conjoint analysis section to provide visibility around leading classes
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs
- To understand the perspective of Key Opinion Leaders’ around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future
- Detailed insights on the unmet need of the existing Hepatic Encephalopathy Drugs Market so that the upcoming players can strengthen their development and launch strategy
Stay Updated with us for Recent Articles