Hepatitis D Market
- Hepatitis D also referred to as “satellite virus,” infects roughly 5% of those with hepatitis B.
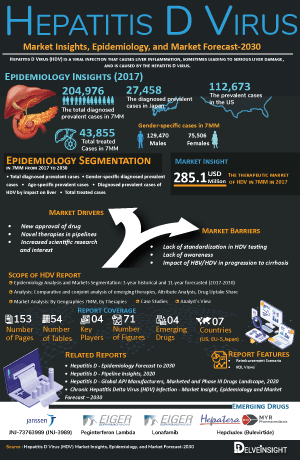
- Among the 7MM, the US accounted for the highest number of diagnosed prevalent cases of hepatitis D virus in 2023. Among these cases, males accounted for a higher number than females.
- The US captured the largest market size of Hepatitis D virus among the 7MM in 2023.
- On 7th September 2023, Eiger BioPharmaceuticals announced the discontinuation of the Phase III LIMT-2 study of peginterferon lambda in patients with chronic hepatitis D. The decision was based on the recommendation of the Data Safety Monitoring Board (DSMB) for the study following its quarterly safety review.
- Currently, treatment strategies for HDV mainly include interferon (IFN)-based therapy. Antiviral therapy with interferon alfa can be considered in patients with chronic hepatitis D virus infection. The treatment course is usually at least 1 year.
- Despite its discovery over four decades ago, there are no United States Food and Drug Administration (US FDA)–approved therapies specifically targeting the hepatitis D virus, leaving patients with limited treatment options.
DelveInsight’s "Hepatitis D Virus Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of hepatitis D, historical and forecasted epidemiology as well as hepatitis D market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
Hepatitis D market report provides current treatment practices, emerging drugs, Hepatitis D market share of individual therapies, and current and forecasted Hepatitis D market size from 2020 to 2034, segmented by seven major markets. The report also covers current Hepatitis D treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Hepatitis D Epidemiology |
Segmented by:
|
|
Hepatitis D key companies |
|
|
Hepatitis D key therapies/drug |
|
|
Hepatitis D Market |
Segmented by:
|
|
Analysis |
|
Hepatitis D Treatment Market
Hepatitis D Overview
Hepatitis D, which is also known as delta hepatitis, is a liver infection caused by the hepatitis D virus. Hepatitis D only occurs in people who are also infected with the hepatitis B virus. Hepatitis D is spread when blood or other body fluids from a person infected with the virus enter the body of someone who is not infected. Hepatitis D can be an acute, short-term infection or become a long-term, chronic infection. Hepatitis D can cause severe symptoms and serious illness that can lead to life-long liver damage and even death. People can become infected with both hepatitis B and hepatitis D viruses at the same time (known as “coinfection”) or get hepatitis D after first being infected with the hepatitis B virus (known as “superinfection”).
Hepatitis D Diagnosis
To accurately diagnose hepatitis D, a blood test is conducted by the doctor to detect anti-hepatitis D antibodies. The presence of these antibodies indicates exposure to the virus. Additionally, if there are suspicions of liver damage, a liver function test is administered. This test evaluates liver health by analyzing levels of proteins, liver enzymes, and bilirubin in the blood. The results of the liver function test determine if the liver is experiencing stress or damage.
Further details related to diagnosis will be provided in the report…
Hepatitis D Treatment
Currently, there are no effective treatments available for either acute or chronic hepatitis D, unlike other forms of hepatitis. Antiviral medications that are commonly used for treating other hepatitis viruses have shown limited effectiveness against HDV. Instead, patients may undergo treatment with high doses of a medication known as interferon for a duration of up to 12 months. However, even after treatment, individuals with hepatitis D may continue to test positive for the virus, emphasizing the importance of preventive measures to curb transmission. In cases where cirrhosis or other forms of liver damage are present, a liver transplant may be necessary. This complex surgical procedure involves replacing the damaged liver with a healthy one obtained from a donor.
Further details related to treatment will be provided in the report...
Hepatitis D Epidemiology
The hepatitis D epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the Total Diagnosed Prevalent cases of HDV, Gender-specific Diagnosed Prevalent Cases of HDV, Age-specific Prevalent Cases of HDV, Diagnosed Prevalent Cases of HDV by Impact on the Liver, and Total Treated Cases of HDV in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In the 7MM, the US accounted for the highest number of diagnosed prevalent cases of Hepatitis D virus in 2023.
- Among the gender-specific cases, males accounted for a higher number of diagnosed prevalent cases than females in the US in 2023.
- Among the age-specific cases, the highest number of cases comprised the age group between 18–34 years in the US in 2023.
- Among EU4 and the UK, Germany accounted for the highest number of diagnosed prevalent cases in 2023.
Hepatitis D Drug Chapters
The drug chapter segment of the Hepatitis D report encloses a detailed analysis of the late-stage (Phase III ) and mid-stage (Phase II/III and Phase II) pipeline drugs. The current key players include Eiger Biopharmaceuticals (Lonafarnib/ Ritonavir), Johnson & Johnson (JNJ-73763989), and others.
The drug chapter also helps understand the Hepatitis D clinical trial details, pharmacological action, agreements and collaborations, and the latest news and press releases. There are no FDA-approved therapies specifically targeting HDV, leaving patients with limited treatment options.
Hepatitis D Marketed Drugs
HEPCLUDEX (Bulevirtide): Gilead Sciences
HEPCLUDEX is an antiviral medicine used to treat chronic (long-term) hepatitis delta virus infection in adults with compensated liver disease (when the liver is damaged but is still able to work) when the presence of viral RNA (genetic material) has been confirmed by blood tests. The active substance in HEPCLUDEX, bulevirtide, works by attaching to and blocking a receptor (target) through which the hepatitis delta and hepatitis B viruses enter liver cells. By blocking the entry of the virus into the cells, HEPCLUDEX limits the ability of HDV to replicate, preventing the spread of the virus in the liver and thereby reducing inflammation. In July 2020, it received conditional approval in Europe. The company has since provided comprehensive information confirming the findings from earlier studies. As a result, the conditional authorization has been switched to a standard one.
|
Product |
Company |
RoA |
Indication |
Approval |
|
HEPCLUDEX (Bulevirtide) |
Gilead Sciences |
Subcutaneous Injection |
Chronic Hepatitis Delta |
2020 (EU) |
Hepatitis D Emerging Drugs
Lonafarnib/ Ritonavir: Eiger Biopharmaceuticals
Lonafarnib is Eiger Biopharmaceuticals' lead program in development as a first-in-class prenylation inhibitor, boosted with ritonavir, for the treatment of hepatitis delta virus infection. Lonafarnib is a well-characterized, orally active inhibitor of farnesyl transferase, an enzyme involved in the modification of proteins through a process called prenylation, a vital process in the life cycle of HDV. Lonafarnib inhibits the farnesylation step of HDV replication inside liver cells and blocks the ability of the virus to multiply. As farnesylation is a host process, not under the control of HDV, and Lonafarnib inhibits farnesylation, hence there is also a potentially higher barrier to resistance with Lonafarnib therapy. Currently, the pivotal Phase III D-LIVR study (NCT03719313) is ongoing and enrolling patients.
JNJ-73763989: Johnson & Johnson
JNJ-73763989 also known as JNJ-3989 is an investigational drug by Janssen Pharmaceuticals. It is a liver-targeted antiviral therapeutic for subcutaneous injection via ribonucleic acid interference mechanism. It is designed to silence all HBV gene products and intervene upstream of the reverse transcription process. The trials are also being conducted by the company for JNJ-73763989 to treat chronic HBV infection. Currently, the company is conducting phase II (NCT04535544), a multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of JNJ-73763989 in HBV-infected patients who are co-infected with HDV.
|
Product |
Company |
Phase |
MOA |
Molecule Type |
ROA |
|
Lonafarnib/ Ritonavir |
Eiger Biopharmaceuticals |
III |
reduces the farnesylation of numerous cellular proteins, including progerin |
Small Molecule |
Oral |
|
JNJ-73763989 |
Johnson & Johnson |
II |
RNA interference |
Small Molecule |
Subcutaneous Injection |
Hepatitis D Market Outlook
Chronic delta hepatitis is a global health problem. Although a smaller percentage of chronic HBV-infected patients are coinfected with the hepatitis delta virus, these patients have a higher risk of an accelerated progression to fulminant “delta hepatitis”, cirrhosis, hepatic decompensation, and hepatocellular carcinoma, putting a financial strain on the healthcare system and increasing the need for a liver transplant. Since its discovery, tremendous efforts have been directed toward understanding the intricate pathogenic mechanisms, discovering the complex viral replication process, the essential replicative intermediates, and cell division-mediated viral spread, which enables virion viability. Bulevirtide and lonafarnib have been shown to effectively inhibit the extracellular spreading pathway by acting as an entry inhibitor and a secretion inhibitor, respectively. HEPCLUDEX is an entry inhibitor, Lonafarnib acts as an oral prenylation inhibitor, and Nucleic acid polymers block viral entry.
Detailed market assessment will be provided in the final report.
Key Findings
- The therapeutic landscape is driven by current treatment practices and the expected launch of emerging therapies.
- Among 7MM, the US is expected to capture the highest market size for the Hepatitis D virus by 2034.
- Larger firms have an edge in the industry because they have the resources and expertise to organize intricate development pathways.
Hepatitis D Pipeline Development Activities
The report provides insights into Hepatitis D clinical trials within Phase III, Phase II/III, and Phase II. It also analyzes key players involved in developing targeted therapeutics. Companies like Eiger Biopharmaceuticals, Johnson & Johnson, and others actively engage in late and mid-stage research and development efforts for Hepatitis D. However, there is a positive outlook for the therapeutics market, with expectations of growth during the forecast period (2024–2034).
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Hepatitis D.
|
KOL Views |
|
“Historically, there have been no licensed treatment options available for NHS patients with chronic hepatitis D in England and Wales. Delivered via subcutaneous injection, the peptide-based therapy can target a virus that can be life-threatening as it causes serious liver damage and liver cancer.” |
|
“Hepatitis D is spreading at a rapid pace even though HBV vaccination plans have existed for a long time. Therefore, it becomes essential to encourage all individuals with chronic hepatitis B to be evaluated for HDV infection.” |
KOL Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Market Access and Reimbursement
Reimbursement is the price negotiation between the manufacturer and payer that allows the manufacturer access to that market. It is provided to reduce the high costs and make essential drugs affordable. In the US healthcare system, both Public and Private health insurance coverage are included. In addition, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, the Children’s Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services and educational programs to aid patients are also present. The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc. In some circumstances, a patient with a serious or life-threatening disease may not be able to participate in a clinical trial. Seeking the use of an investigational medication outside of a clinical trial is permitted by the FDA and commonly referred to as Compassionate Use or Expanded Access. Eiger BioPharmaceuticals’ Expanded Access Policy is intended to comply with US FDA requirements for any such use or access. Eiger considers many factors when evaluating a request for expanded access to an investigational medicine, such as (but not limited to) the strength of the clinical data, the benefit-risk profile, the impact on the clinical development program, the phase of development, and probability and timing of regulatory approval.
Detailed market access and reimbursement assessment will be provided in the final report...
Scope of the Hepatitis D Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of Hepatitis D, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Hepatitis D market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive Hepatitis D.
Hepatitis D Report Insights
- Hepatitis D Patient Population
- Hepatitis D Therapeutic Approaches
- Hepatitis D Pipeline Analysis
- Hepatitis D Market Size and Trends
- Existing and Future Market Opportunity
Hepatitis D Report Key Strengths
- Eleven Years Forecast
- The 7MM Coverage
- Hepatitis D Epidemiology Segmentation
- Key Cross Competition
- Hepatitis D Drugs Uptake
- Key Hepatitis D Market Forecast Assumptions
Hepatitis D Report Assessment
- Current Hepatitis D Treatment Practices
- Hepatitis D Unmet Needs
- Hepatitis D Pipeline Product Profiles
- Hepatitis D Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Hepatitis D Market Drivers
- Hepatitis D Market Barriers
FAQs
- What was the Hepatitis D market size, the market size by therapies, market share (%) distribution in 2023, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for Hepatitis D?
- What are the disease risks, burdens, and unmet needs of Hepatitis D? What will be the growth opportunities across the 7MM concerning the patient population with Hepatitis D?
- What are the current options for the treatment of Hepatitis D? What are the current guidelines for treating Hepatitis D in the 7MM?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What is the patient share in Hepatitis D?
Reasons to Buy Hepatitis D Market Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving Hepatitis D
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis ranking of class-wise potential current and emerging therapies under the analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.


-02.png)
-01-01.png)
-Infection-Market-Outlook.png&w=256&q=75)