HER2-low Cancers Epidemiology Forecast
Key Highlights
- HER2-low is a newly defined category characterized by HER2 expression of 1+ or 2+ by immunohistochemistry (IHC) and the absence of HER2 gene amplification, as measured by in situ hybridization (ISH) and it is commonly observed in various solid tumors, with incidence rates ranging from 15% to 65% across different types of cancer.
- As the population ages, the prevalence of breast cancer in the elderly is rising, with HER2-low cases comprising over 50% of traditional breast cancers.
- In the 7MM, in 2024, among all the HER2-low cancers indications, breast cancer accounted for the highest number of incident cases, while ovarian cancer occupied the bottom of the ladder.
- As per the estimates, the treatment eligible pool (combined first and second line above) for HER2-low cancers in the 7MM is expected to increase by 2034 from 500,000 in 2024 during the study period (2020–2034).
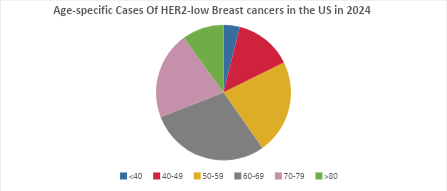
- The highest number of cases of HER2-low breast cancer were seen in the 60-69 age group, while the lowest were in the <40 age group.
- The incident cases of HER2-low breast cancers were estimated at 64,000 in 2024, projected to increase by 2034. Our estimates indicate the US had the highest incident cases among the 7MM.
DelveInsight’s “HER2-low Cancers – Epidemiology Forecast – 2034” report delivers an in-depth understanding of HER2-low cancers, historical and forecasted epidemiology in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
HER2-low Cancers Understanding
HER2-low Cancers Overview
HER2 is a transmembrane tyrosine kinase receptor that is a member of the epidermal growth factor receptor family and is encoded by the ERBB2 gene. HER2 mediates cell growth, differentiation, and survival. HER2 status is assayed using standard molecular methods based on the assessment of HER2 protein overexpression by IHC and estimation of HER2 gene amplification by FISH. HER2-low is defined as a HER2 immunohistochemical expression of 1+ or 2+ without amplification by ISH (IHC score of 1+ or 2+ with FISH-negative).
The major signaling pathways mediated by HER2/neu involve the Mitogen-activated Protein Kinase (MAPK) pathway and the Phosphatidylinositol 3-kinase (PI3K) pathway. As a critical gene for cell survival, HER2/neu gene amplification and protein overexpression lead to malignant transformation. HER2 expression is found in different types of cancer, including colorectal, ovarian, endometrial, bladder, gastric, and BTC, with the majority being breast cancer.
HER2-low Cancer Diagnosis
The diagnosis of HER2-low cancers presents significant challenges due to the complexity of identifying low HER2 expression levels, which is influenced by multiple methodological and analytical variables. These factors can impact the sensitivity and reproducibility of testing, particularly in distinguishing between HER2-low (immunohistochemistry [IHC] score 1+) and HER2-zero (IHC score 0), which also includes the subset classified as HER2 ultra-low (IHC score 0 with incomplete and faint staining in ≤10% of tumor cells). The primary approach to diagnosing HER2-low expression involves HER2 testing through in situ hybridization (ISH) and IHC, with the Ventana anti-HER2 assay being the only FDA-approved testing method.
HER2 testing is a standard procedure for all new breast cancer diagnoses, as well as in case of tumor progression and/or residual tumor after neoadjuvant treatment. This analysis relies on IHC and ISH. In particular, IHC detects the expression and intensity of HER2 protein on the cell membrane by a three-tier scoring system (from score 0 to score 3+). In contrast, ISH detects the presence of gene amplification using HER2 and CEP17 probes.
VENTANA anti-HER2 (4B5) antibody is a rabbit monoclonal antibody approved by the FDA, which binds to HER2 in Formalin-fixed, Paraffin-embedded (FFPE) tissue sections. The specific antibody can be localized by either a biotin-conjugated secondary antibody formulation that recognizes rabbit immunoglobulins, followed by the addition of a streptavidin-Horseradish Peroxidase conjugate (iVIEW DAB Detection Kit) or a secondary antibody-HRP conjugate (ultraView Universal DAB Detection Kit).
Further details related to diagnosis will be provided in the report…
Explore detailed insights into market forecast, emerging therapies, market trends, and key players shaping the Heparin-Induced Thrombocytopenia landscape - Her2 Low Cancers Market Report
HER2-low Cancers Epidemiology
The HER2-low cancers epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total targeted patient pool of selected indications in HER2-low cancers, treatment eligible pool of selected indications for HER2-low cancers, total incident cases of HER2-low breast cancers, age-specific cases of HER2-low breast cancers, and stage-specific cases of HER2-low breast cancers in the 7MM covering the US, EU4 (Germany, France, Italy, Spain) and the UK, and Japan from 2020 to 2034.
- The incident cases of HER2-low breast cancers were estimated at nearly 19,000 in 2024, projected to reach xx, xxx by 2034 in Japan.
- In EU4 and the UK, the age group between 60–69 accounted for the highest proportion, followed by the 50–59 age group, while cases among those under 40 remain the lowest. In 2024, there were almost 15,000, 12,000, and 2,000 in the age groups 60–69 years, 50–59 years, and <40 respectively.
- In the US in 2024, the total cases of HER2-low breast cancers in Stage I, Stage II, Stage III, and Stage IV were identified to be nearly 38,000, 17,000, 5,000, and 2,000, which is likely to increase in Stage I, Stage II, Stage III and Stage IV, respectively by 2034.
- In the UK in 2024, HER2-low expression was seen highest in breast cancer, i.e., 55% out of almost 58,000 cases, and least in ovarian cancer, i.e., 17% out of nearly 7,700 cases.
HER2-low Cancers Report Insights
HER2-low Cancers Report Insights
- Patient population
- Country-wise epidemiology distribution
HER2-low Cancers report key strengths
- Ten-year forecast
- 7MM coverage
- HER2-low cancers epidemiology segmentation
FAQs
- What are the disease risks, burdens, and unmet needs of HER2-low cancers? What will be the growth opportunities across the 7MM concerning the patient population with HER2-low cancers?
- What is the historical and forecasted HER2-low cancers patient pool in the US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Reasons to Buy
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand key opinion leaders’ perspectives around the diagnostic challenges to overcome barriers in the future.
- Detailed insights on various factors hampering disease diagnosis and other existing diagnostic challenges.