Hypofibrinogenemia Market Summary
- The Hypofibrinogenemia Market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected market launch of therapies, and raised awareness.
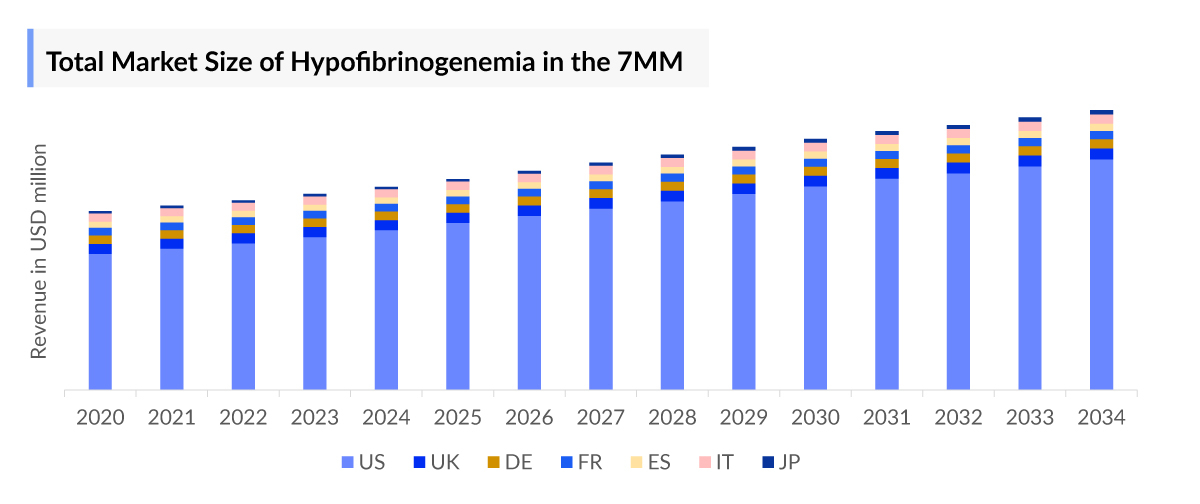
- The Hypofibrinogenemia Market Size in the 7MM was ~USD 200 million in 2023.
Hypofibrinogenemia Market Insights and Trends
- Hypofibrinogenemia is a condition characterized by abnormally low levels of fibrinogen, a protein essential for blood clotting.
- It can be either congenital (inherited) or acquired. In congenital hypofibrinogenemia, bleeding episodes are the main symptoms, whereas acquired hypofibrinogenemia is more frequently asymptomatic.
- Few approved Hypofibrinogenemia Drugs are- RiaSTAP, Fibryna, FibCLOT (Clottafact), and others.
- Hypofibrinogenemia Pipeline is not so robust but possesses a potential drug, i.e., AdFirst (BT-524).
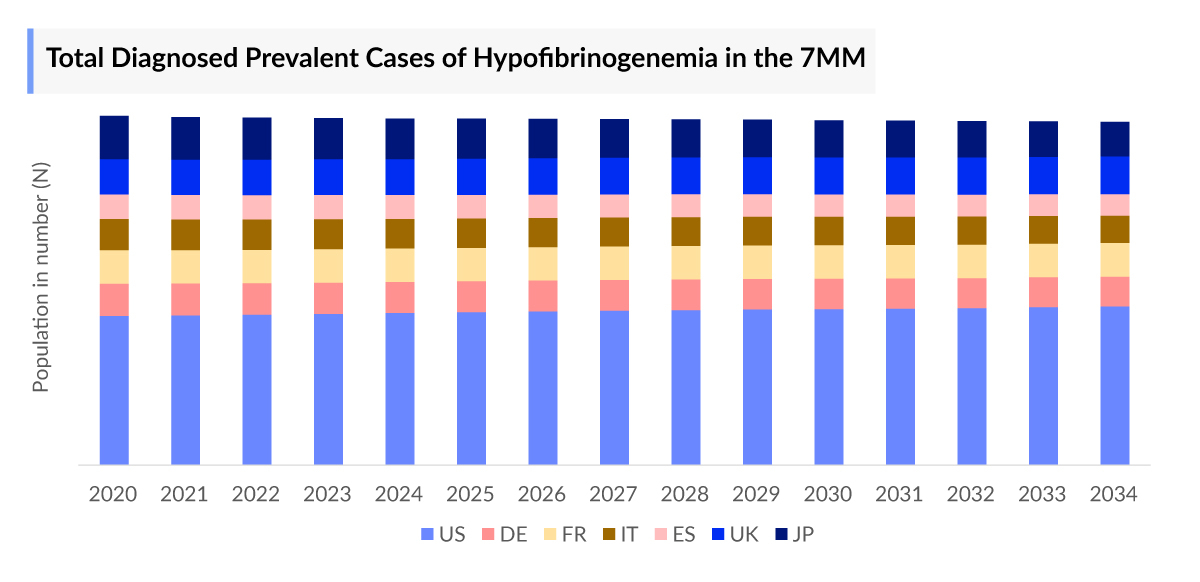
- The Hypofibrinogenemia Cases in the 7MM in 2023 were ~11,000 cases, and the United States accounted for the highest with ~7,000 cases.
- Japan had the second highest number of cases, contributing approximately 1,300 cases, which accounts for about 10% of the total cases in the 7MM.
- The United States accounts for the largest Hypofibrinogenemia Treatment Market Size, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- In February 2024, Biotest announced that the AdFirst phase III trial showed that Fibrinogen concentrate is non-inferior to standard care in reducing intraoperative blood loss for patients with acquired fibrinogen deficiency. The mean blood loss was 1,444 mL with Fibrinogen concentrate versus 1,735 mL with standard care, resulting in a 291 mL reduction.
Request for Unlocking the Sample Page of the "Hypofibrinogenemia Treatment Market"
Factors Affecting the Hypofibrinogenemia Market Growth
Increasing Awareness and Diagnosis of Rare Bleeding Disorders
Growing awareness among healthcare professionals and patients regarding rare coagulation disorders, including congenital and acquired hypofibrinogenemia, is driving early diagnosis and management. The expansion of diagnostic programs and improved screening for fibrinogen deficiencies have positively influenced patient identification rates.
Advancements in Diagnostic Technologies
The development of high-sensitivity fibrinogen assays, genetic testing, and next-generation sequencing (NGS) has significantly enhanced diagnostic accuracy. These technological improvements enable early detection and differentiation between congenital and acquired hypofibrinogenemia, supporting better disease management and market expansion.
Introduction of Recombinant and Plasma-Derived Fibrinogen Concentrates
The availability and increasing adoption of fibrinogen replacement therapies, such as human fibrinogen concentrates (RiaSTAP, Fibryga) and recombinant fibrinogen, have transformed the treatment landscape. These targeted therapies reduce dependence on cryoprecipitate or fresh frozen plasma (FFP), improving safety and clinical outcomes.
Rising Incidence of Acquired Coagulopathies and Trauma Cases
A growing number of acquired hypofibrinogenemia cases arising from conditions like trauma, postpartum hemorrhage, liver disease, or disseminated intravascular coagulation (DIC) are driving the demand for fibrinogen replacement therapies. The increasing frequency of complex surgical and trauma cases also contributes to market growth.
Ongoing Research and Clinical Trials
Continuous research into the genetic basis of congenital fibrinogen disorders and clinical evaluation of novel fibrinogen formulations has expanded therapeutic options. Collaborative studies and orphan drug designations by regulatory bodies are further supporting innovation in this domain.
Government Support and Orphan Drug Incentives
Regulatory incentives such as orphan drug status, fast-track designations, and reimbursement support for rare diseases encourage pharmaceutical companies to invest in the development of therapies for hypofibrinogenemia, thereby driving market growth.
DelveInsight's “Hypofibrinogenemia Treatment Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Hypofibrinogenemia, historical and forecasted epidemiology as well as the Hypofibrinogenemia market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Hypofibrinogenemia Treatment Market Report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Hypofibrinogenemia market size from 2020 to 2034. The report also covers current Hypofibrinogenemia Treatment Market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Hypofibrinogenemia Epidemiology |
Segmented By:
|
|
Hypofibrinogenemia Companies |
|
|
Hypofibrinogenemia Drugs |
|
|
Hypofibrinogenemia Market |
Segmented by
|
|
Analysis |
|
Hypofibrinogenemia Treatment Market
Hypofibrinogenemia is a condition characterized by abnormally low levels of fibrinogen, a protein essential for blood clotting. It can be either congenital (inherited) or acquired. In congenital hypofibrinogenemia, bleeding episodes are the main symptoms, whereas acquired hypofibrinogenemia is more frequently asymptomatic. Acquired hypofibrinogenemia is most commonly caused by hemodilution and consumption of clotting factors, often encountered in situations like massive hemorrhage, liver disease, and disseminated intravascular coagulation.
Hypofibrinogenemia diagnosis typically involves a combination of coagulation tests and fibrinogen assays. Prolonged prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), and reptilase time (RT) are common findings, with the degree of prolongation depending on the severity of hypofibrinogenemia. The Clauss fibrinogen assay, which measures the functional activity of fibrinogen, is the most widely used method to quantify fibrinogen levels. Fibrinogen antigen assays, which measure the total amount of fibrinogen, are also available. In some cases, genetic testing may be necessary to identify the specific mutations causing congenital hypofibrinogenemia.
Hypofibrinogenemia Treatment
The treatment of hypofibrinogenemia depends on the severity of bleeding and the underlying cause. For congenital hypofibrinogenemia with clinically significant bleeding, fibrinogen concentrate is administered to raise levels to 100-150 mg/dL. In cases of severe bleeding, such as intracranial hemorrhage, higher target levels of 150-200 mg/dL are necessary. For acquired hypofibrinogenemia, the underlying cause must be addressed, and supportive measures like fibrinogen replacement therapy may be required. Fibrinogen replacement can be achieved using cryoprecipitate, fresh frozen plasma (FFP), or specific fibrinogen concentrates. Monitoring of fibrinogen levels is crucial to ensure adequate replacement and to guide further treatment.
Hypofibrinogenemia Epidemiology
The Hypofibrinogenemia epidemiology chapter in the report provides historical as well as forecasted data in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Hypofibrinogenemia epidemiology is segmented with detailed insights into Total Hypofibrinogenemia Cases, Hypofibrinogenemia Type-Specific Cases, and Hypofibrinogenemia Acquired Hypofibrinogenemia Cases.
Key Findings from Hypofibrinogenemia Epidemiological Analyses and Forecast
- As per DelveInsight estimates, in 2023, there were ~700 cases of Congenital Hypofibrinogenemia and ~6,000 cases of Acquired Hypofibrinogenemia in the US.
- In 2023, there were ~2,100 cases of Liver surgery and ~1,900 cases of cardiovascular surgery. Assessments as per DelveInsight’s analysts show that the overall cases of Acquired Hypofibrinogenemia is subjected to increase in the forecasted period.
- Among EU4 and the UK, Germany accounted for the highest cases of Hypofibrinogenemia ~800 cases in 2023.
Hypofibrinogenemia Drugs Analysis
The drug chapter segment of the Hypofibrinogenemia Treatment Market Report encloses a detailed analysis of Hypofibrinogenemia marketed drugs and late-stage (Phase III and Phase II) Hypofibrinogenemia Pipeline Drugs. It also deep dives into the pivotal Hypofibrinogenemia Clinical Trials details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Hypofibrinogenemia Marketed Drugs
-
RiaSTAP: CSL Behring
RiaSTAP is a heat-treated, lyophilized fibrinogen (coagulation factor I) powder made from pooled human plasma. It is manufactured from cryoprecipitate into a glycine precipitate, which is then further purified by multiple precipitation/adsorption steps. RiaSTAP is indicated for the treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia in the US and Europe. Approval was based on a pivotal phase II prospective, open-label pharmacokinetic (PK) and safety study using maximum clot firmness (MCF) as a surrogate endpoint for hemostatic efficacy. Outside the United States, RiaSTAP is marketed under the trade name of Haemocomplettan.
-
Fibryna: Octapharma
FIBRYNA (Octafibrin) is a human plasma-derived, sterile, purified, virus-inactivated, and nano filtered (20 nm) fibrinogen concentrate. It has a fixed, labeled content of fibrinogen and is double pathogen-inactivated. It supplied as a lyophilized powder that can be stored at room temperature and reconstituted within minutes. The reconstituted product is stable at room temperature for 24 h. Conversely, cryoprecipitate must be thawed before use and must be used within 4 h of thawing. The amount of fibrinogen in cryoprecipitate can vary considerably from preparation to preparation. Also, the pooling of plasma during the preparation of cryoprecipitate carries a potential risk of infectious transmission.
The product is indicated for the treatment of acute bleeding episodes in adults and adolescents with congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia in the US and Europe. Later on, in various European countries, the product got extended approval for acquired fibrinogen deficiency.
Note: Detailed current therapies assessment will be provided in the full report of Hypofibrinogenemia
Hypofibrinogenemia Emerging Drugs
-
AdFIrst (BT-524): Biotest AG
BT-524 is a human fibrinogen concentrate purified from human plasma. Fibrinogen is a plasma protein produced by the liver, which is an important component of blood clotting. A deficiency of autologous fibrinogen means that the blood’s ability to clot is impaired, which leads to a greatly increased risk of bleeding and, additionally, to a delay in bleeding cessation. In the case of congenital fibrinogen deficiency, patients can produce no or only insufficient fibrinogen. In acquired fibrinogen deficiency, on the other hand, patients lose fibrinogen because of heavy bleeding, for example, due to severe injuries and surgery. In both cases, fibrinogen must be supplied again to stop bleeding. The study aimed to evaluate the pharmacokinetics, efficacy, and safety of BT524 in 36 participants with specific conditions. BT524 was administered intravenously, and its effects were monitored over 14 days following a single dose. Currently in Phase III for Congenital Fibrinogen Deficiency and Acquired Fibrinogen Deficiency.
Hypofibrinogenemia Market Outlook
Key Hypofibrinogenemia Companies, such as Biotest AG, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Hypofibrinogenemia.
- DelveInsight estimates show that the United States accounted for the highest Hypofibrinogenemia Treatment Market Size in 7MM of ~ USD 150 million.
- Among EU4 and the UK, Germany has the highest Hypofibrinogenemia Treatment Market Size ~USD 10 million in 2023.
- In the US, cryoprecipitate generated the highest revenue of ~USD 70 million for congenital hypofibrinogenemia and ~10 million for vvv, in 2023.
- BT-524 (Biotest) is the only emerging therapy in the developmental phase for the treatment of Congenital and Acquired Hypofibrinogenemia.
Hypofibrinogenemia Drugs Uptake
This section focuses on the uptake rate of potential Hypofibrinogenemia drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data, along with an order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Hypofibrinogenemia Clinical Trials Analysis
The Hypofibrinogenemia Market Report provides insights into different therapeutic candidates in the Phase III and Phase II stages. It also analyzes key Hypofibrinogenemia Companies involved in developing targeted therapeutics.
Hypofibrinogenemia Pipeline Development Activities
The Hypofibrinogenemia Therapeutics Market Report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Hypofibrinogenemia emerging therapies.
Latest KOL Views on Hypofibrinogenemia
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 40+ KOLs to gather insights; however, interviews were conducted with 25+ KOLs in the 7MM. Centers such as The hematologist of John Hopkins Centre of Hematology and Oncology, Brigham and Women’s Hospital, etc., were contacted. Their opinion helps understand and validate current and emerging treatment patterns of Hypofibrinogenemia. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Hypofibrinogenemia Therapeutics Market and the unmet needs.
What KOLs are saying on Hypofibrinogenemia Patient Trends?
|
Region |
KOL Views |
|
United States |
”Either we use the cryoprecipitate which is produced by thawing the fresh frozen plasma or fibrinogen concentrates. Both are equally effective in nature. But most of the patients here in the US, do go for cryoprecipitate because it is less expensive than fibrinogen concentrates.” |
|
United Kingdom |
“The most common treatment used are the fibrinogen concentrates as compared to plasma concentrates. Fibrinogen concentrates are available more easily in the hospitals.” |
|
France |
"Cryoprecipitate is used majority of the times because it is easily accessible and the price is also not so high as compared to fibrinogen concentrates. However the patient choice is preferred, if fibrinogen concentrates are requested, the same can be arranged by the management.” |
Hypofibrinogenemia Therapeutics Market: Qualitative Analysis
We perform Qualitative and Hypofibrinogenemia Therapeutics Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, Terminal Elimination Half-life (t1/2) for Fibrinogen Antigen, one of the most important primary outcome measures, is compared to Maximum Concentration (Tmax) for Fibrinogen Antigen.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Hypofibrinogenemia Therapeutics Market Access and Reimbursement
The Hypofibrinogenemia Therapeutics Market Report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Hypofibrinogenemia Treatment Market Report
- The Hypofibrinogenemia treatment market report covers a segment of key events, an executive summary, a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current Hypofibrinogenemia Treatment Market Landscape.
- A detailed review of the Hypofibrinogenemia Therapeutics Market, historical and forecasted Hypofibrinogenemia Treatment Market Size, Hypofibrinogenemia Drugs Market Share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Hypofibrinogenemia Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Hypofibrinogenemia Drugs Market.
Hypofibrinogenemia Market Report Insights
- Patient-based Hypofibrinogenemia Market Forecasting
- Therapeutic Approaches
- Hypofibrinogenemia Pipeline Drugs Analysis
- Hypofibrinogenemia Treatment Market Size and Trends
- Existing and Future Hypofibrinogenemia Drugs Market Opportunity
Hypofibrinogenemia Market Report Key Strengths
- 11 Years Hypofibrinogenemia Market Forecast
- 7MM Coverage
- Hypofibrinogenemia Epidemiology Segmentation
- Inclusion of country-specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Hypofibrinogenemia Drugs Uptake
- Key Hypofibrinogenemia Market Forecast Assumptions
Hypofibrinogenemia Market Report Assessment
- Current Hypofibrinogenemia Treatment Market Practices
- Hypofibrinogenemia Unmet Needs
- Hypofibrinogenemia Pipeline Drugs Analysis
- Hypofibrinogenemia Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs by Hypofibrinogenemia Market Report
- What is the growth rate of the 7MM Hypofibrinogenemia treatment market?
- What was the Hypofibrinogenemia treatment market size, the Hypofibrinogenemia market size by therapies, Hypofibrinogenemia drugs market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Hypofibrinogenemia?
- How many Hypofibrinogenemia companies are developing therapies for the treatment of Hypofibrinogenemia?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy Hypofibrinogenemia Market Report
- The Hypofibrinogenemia Therapeutics Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Hypofibrinogenemia Drugs Market.
- Insights on patient burden/disease Hypofibrinogenemia Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Hypofibrinogenemia Drugs Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Hypofibrinogenemia Drugs Market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Hypofibrinogenemia Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for Recent Articles