Inflammation and Pain Post Cataract Surgery Market
- The inflammation and pain post-cataract surgery market is expected to witness significant growth during the forecast period (2024–2034). The increase in market size is a direct consequence of increased awareness about the disease among doctors, increased prevalence of ocular disorders, and ongoing research and development of new treatments.
- The current inflammation and pain post-cataract surgery market is dominated by companies like Ocular Therapeutix, Alcon, and Eyenovia with products like DEXTENZA, INVELTYS, and APP13007 in the market for inflammation and pain post-cataract surgery.
- To propel the market in the coming years, companies like VivaVision Biotech and Oculis have assets like VVN461 and OCS-01 for inflammation and pain post-cataract surgery. With the anticipated approval of emerging therapies currently in development in the coming years the overall Inflammation and pain post-cataract surgery therapeutics market is expected to grow at a significant CAGR over the forecast period [2024–2034].
DelveInsight’s comprehensive report titled “Inflammation and Pain Post-Cataract Surgery Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Inflammation and pain post-cataract surgery. The report presents historical and projected epidemiological data covering the total diagnosed prevalent cases of cataract, total cases of cataract surgery, total cases of inflammation and pain (post-cataract surgery), and treated cases of inflammation and pain (post-cataract surgery). In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing treatment practices and unmet medical requirements in inflammation and pain post-cataract surgery. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
Inflammation and Pain Post-Cataract Surgery Overview
Cataracts are developed when the lens of your eye, a small transparent disc, develops cloudy patches. The natural crystalline lens of the eye is clear which allows light to be focused clearly on the retina. With aging, this lens loses its transparency and gradually becomes clouded thereby impairing vision. Surgery to replace the cloudy lens is the only way to improve the eyesight. For most people, cataract surgery goes smoothly.
The physical trauma associated with cataract surgery, including disruption of the blood-aqueous barrier (BAB), can induce an inflammatory response and the release of inflammatory mediators such as prostaglandins and leukotrienes from arachidonic acid. Despite surgical advances, post-cataract surgery inflammation is still a common cause of patient discomfort, delayed recovery, and reduced visual outcome. Symptoms may include pain or discomfort, decreased vision, photophobia (sensitivity to light), redness, and swelling.
Inflammation and Pain Post-Cataract Surgery Diagnosis and Treatment Algorithm
To diagnose these conditions, ophthalmologists may perform various tests and evaluations, such as ocular examination to assess inflammation in the anterior chamber, measurement of intraocular pressure, evaluation of visual acuity, assessment of the presence of complications like cystoid macular edema, monitoring for signs of infection or other inflammatory etiologies.
Inflammation after cataract surgery, which can be persistent, remains an undesirable consequence despite many advances in surgical techniques. Corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) have traditionally been used to treat inflammation, prophylactically as well as post-operatively, but there are no established guidelines for the treatment of inflammation induced by cataract surgery. Topical antibiotics are used to prevent the development of postoperative endophthalmitis.
The most prescribed drugs are the 4th generation fluoroquinolones, gatifloxacin, and moxifloxacin. Topical NSAIDs have also been used in the pre-operative, peri-operative, and post-operative settings for their multifaceted effect. Current research shows that NSAIDs are effective in maintaining pupillary dilation intra-operatively as well as reducing the incidence of cystoid macular edema (CME) when combined with corticosteroids postoperatively.
Inflammation and Pain Post-Cataract Surgery Epidemiology
The epidemiology section of the Inflammation and pain post-cataract surgery market report offers information on the patient populations, including historical and projected trends for each of the seven major markets.
Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of inflammation and pain post-cataract surgery. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
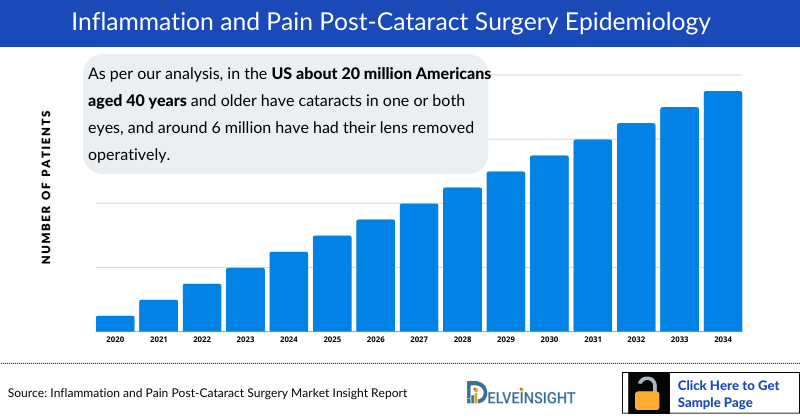
- As per our analysis, in the US about 20 million Americans aged 40 years and older have cataracts in one or both eyes, and around 6 million have had their lens removed operatively. As per our analysis, chronic or recurrent inflammation, including cystoid macular edema, occurs in about 0.1% to 2% of patients following routine cataract surgery.
- As per analysis in 2019, 2,200 cataract surgeries were performed in the US. Of these cases, around 40 eyes had post-operative iritis lasting longer than 1 month.
- Based on our analysis, cataract surgery is the most commonly performed elective surgical procedure in the UK with around 330, 000 cataract operations performed per year in England alone.
Inflammation and Pain Post-Cataract Surgery Market Outlook
Inflammation and pain are becoming common complications post-cataract surgery. Many researchers and industries are working to develop treatments for post-cataract surgery inflammation and pain. The combination of topical NSAIDs and corticosteroids appears to be the most effective approach to control post-cataract surgery inflammation and pain.
If a patient is diagnosed with chronic or recurrent postoperative inflammation 6 to 8 weeks after cataract surgery, then the postoperative steroid is typically restarted. If the inflammation is severe, then dosing may be as frequent as every 2 hours for a few days before being tapered. If the inflammation is mild to moderate, then the steroid is administered four times per day.
The effects of topical corticosteroids on postoperative outcomes include decreasing post-surgical incidence of CME and reducing inflammation such as lid edema, lid injection, conjunctival injection, corneal edema, ciliary flush, and anterior chamber cells. Of note, corticosteroids are particularly effective in controlling inflammation in patients with uveitis. Evidence suggests that NSAIDs and corticosteroids are synergistic in the prevention of CME.
Topical antibiotics are also given to prevent the development of postoperative endophthalmitis. The most recommended drugs are the 4th generation fluoroquinolones, gatifloxacin, and moxifloxacin. These provide optimal coverage for the most implicated pathogens: coagulase-negative staphylococci, staphylococcus aureus, and streptococci.
Four topical ocular NSAIDS are currently approved by the US FDA for the treatment of postoperative inflammation after cataract surgery. They are VOLTAREN (diclofenac sodium ophthalmic solution), ACULAR (ketorolac tromethamine ophthalmic solution), NEVANAC (nepafenac ophthalmic suspension), XIBROM (bromfenac ophthalmic solution).
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the inflammation and pain post-cataract surgery market in the 7MM is expected to change significantly during the study period 2020–2034.
Inflammation and Pain Post-Cataract Surgery Drug Chapters
Marketed Inflammation and Pain Post Cataract Surgery Drugs
DEXTENZA (dexamethasone): Ocular Therapeutix
DEXTENZA (dexamethasone) is the first US FDA-approved intracanalicular insert, a novel route of administration that delivers the drug to the surface of the eye without the need for eye drops. It is a preservative-free, resorbable hydrogel insert that delivers 0.4mg of dexamethasone to treat post-surgical ocular inflammation and pain for up to 30 days with a single administration. DEXTENZA (dexamethasone) originally received the US FDA approval in November 2018 for the treatment of ocular pain following ophthalmic surgery.
INVELTYS (loteprednol etabonate ophthalmic suspension): Alcon
INVELTYS (loteprednol etabonate ophthalmic suspension) 1% is indicated for the treatment of post-operative inflammation and pain following ocular surgery. The mechanism of action of INVELTYS (loteprednol etabonate ophthalmic suspension) involves its function as a corticosteroid that inhibits the inflammatory response to various inciting agents. INVELTYS was approved by the US FDA in August 2018.
APP13007 (clobetasol propionate): Eyenovia
APP13007 (clobetasol propionate) is the first product developed using Formosa’s proprietary APNT nanoparticle formulation platform. When used for the treatment of post-operative inflammation and pain following ocular surgery, APP13007 acts by inhibiting edema, fibrin deposition, capillary dilation, leukocyte migration, and capillary proliferation associated with inflammation. APP13007 was approved by the US FDA in March 2024.
|
Drug |
MoA |
RoA |
Company |
|
DEXTENZA (dexamethasone) |
Corticosteroids which inhibit proinflammatory cytokines |
Intracanalicular |
Ocular Therapeutix |
|
INVELTYS (loteprednol etabonate ophthalmic suspension) |
Altered glucose and antioxidant metabolism and reduced liposomal storage |
Ophthalmic |
Alcon |
|
APP13007 (clobetasol propionate) |
Corticosteroid with anti-inflammatory properties |
Ophthalmic |
Eyenovia |
|
XXX |
XX |
X |
XXX |
Note: Detailed marketed therapies assessment will be provided in the final report....
Emerging Inflammation and Pain post-cataract Surgery Drugs
VVN461: VivaVision Biotech
VVN461 is a potent JAK1 immunomodulator independently developed by VivaVision Biotech, and there is increasing evidence showing that the JAK-STAT signaling pathway is essential for inflammation and immune response. VVN461 can inhibit multiple inflammatory cytokine pathways with high activity to treat postoperative inflammation of cataracts. The results of human pharmacokinetic studies showed that VVN461 ophthalmic solution had low exposure in plasma, indicating that VVN461 ophthalmic solution had a high safety profile due to low systemic toxicity while exerting local anti-inflammatory effects. VVN461 is under clinical development by VivaVision Biotech for the treatment of Inflammation and Pain Post Cataract Surgery. In November 2023, the US FDA approved IND application for the Phase II clinical trial of VVN461.
OCS-01: Oculis
Leveraging Oculis’ proprietary OPTIREACH technology, OCS-01 is a novel, high-concentration (15 mg/ml), topical formulation of dexamethasone. The optireach solubilizing formulation technology addresses the main limitations of conventional eye drops by improving the solubility of lipophilic drugs, increasing the residence time on the eye surface and thereby, enabling less frequent administration for front-of-the-eye and the drug passage from the eye surface to the posterior segment for back-of-the-eye diseases. OCS-01 is a potential once-daily treatment for Post-cataract Surgery Inflammation. OCS-01, the first investigational eye drop designed for front- and back-of-the-eye, met both primary endpoints in the Phase 3 OPTIMIZE-1 trial with a once-daily regimen for treating inflammation and pain following cataract surgery. The trial results showed OCS-01’s superiority in reducing inflammation and pain vs. vehicle as well as a favorable safety profile. OCS-01 is under clinical development by Oculis and is currently in phase III OPTIMIZE-2 trial for the treatment of inflammation and pain post cataract surgery.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
VVN461 |
Janus kinase inhibitor. |
Ophthalmic |
VivaVision Biotech |
II |
|
OCS-01 |
Anti-inflammatory |
Ophthalmic |
Oculis |
III |
|
XXX |
XX |
X |
XXX |
X |
Note: Detailed emerging therapies assessment will be provided in the final report....
Inflammation and Pain Post-Cataract Surgery Market Segmentation
DelveInsight’s ‘Inflammation and Pain Post-Cataract Surgery Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Inflammation and pain post-cataract surgery market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Inflammation and Pain Post-Cataract Surgery Market Size by Countries
The inflammation and pain post-cataract surgery market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2022, the United States held a significant share of the overall 7MM (Seven Major Markets) Inflammation and pain post-cataract surgery market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Country-wise Market Size Distribution of Inflammation and Pain Post-Cataract Surgery
Inflammation and Pain Post-Cataract Surgery Market Size by Therapies
Inflammation and pain post-cataract surgery Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034.
One of the emerging drugs anticipated to launch during the forecast period is OCS-01 by Oculis.
Market Share Distribution of Inflammation and Pain Post-Cataract Surgery by Therapies in 2034
Note: Detailed market segment assessment will be provided in the final report....
Inflammation and Pain Post-Cataract Surgery Drugs Uptake
This section focuses on the sales uptake of potential inflammation and pain post-cataract surgery drugs that have recently been launched or are anticipated to be launched in the inflammation and pain post-cataract surgery market between 2020 and 2034. It estimates the market penetration of Inflammation and pain post-cataract surgery drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Inflammation and pain post-cataract surgery market.
The emerging Inflammation and pain post-cataract surgery therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Inflammation and pain post-cataract surgery market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report...
Inflammation and Pain Post-Cataract Surgery Market Access and Reimbursement
DelveInsight’s ‘Inflammation and pain post-cataract surgery – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Inflammation and pain post-cataract surgery. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Inflammation and pain post-cataract surgery market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the inflammation and pain post-cataract surgery domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Inflammation and pain post-cataract surgery market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Inflammation and pain post-cataract surgery unmet needs.
Inflammation and Pain Post-Cataract Surgery: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Farrer Park Hospital, the University of Michigan's W. K. Kellogg Eye Center, and NHS hospitals among others.
“While pain following cataract surgery is typically mild, some patients still experience discomfort. There's a need for more effective pain management options that provide rapid relief without significant side effects, especially for patients who are sensitive to standard analgesics.”
“More inflammation occurs in patients with longer duration of surgery, a dense cataract that requires more ultrasonic energy to break up, more fluidic flow during surgery, complications during surgery, retained lens material, younger age (younger patients have more inflammation typically than older patients), and a genetic variation of inflammatory response”
Note: Detailed assessment of KOL Views will be provided in the full report on Inflammation and pain post-cataract surgery....
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Inflammation and pain post-cataract surgery Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Inflammation and Pain Post-Cataract Surgery Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for Inflammation and pain post-cataract surgery. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Inflammation and pain post-cataract surgery therapies.
Inflammation and Pain Post-Cataract Surgery Report Insights
- Inflammation and Pain Post-Cataract Surgery Patient Population
- Therapeutic Approaches
- Inflammation and Pain Post-Cataract Surgery Pipeline Analysis
- Inflammation and Pain Post-Cataract Surgery Market Size and Trends
- Inflammation and Pain Post-Cataract Surgery Market Opportunities
- Impact of Upcoming Therapies
Inflammation and Pain Post-Cataract Surgery Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Inflammation and Pain Post-Cataract Surgery Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Inflammation and Pain Post-Cataract Surgery Market
- Inflammation and Pain Post-Cataract Surgery Drugs Uptake
Inflammation and Pain Post-Cataract Surgery Report Assessment
- Inflammation and Pain Post-Cataract Surgery Current Treatment Practices
- Unmet Needs
- Inflammation and Pain Post-Cataract Surgery Pipeline Product Profiles
- Inflammation and Pain Post-Cataract Surgery Market Attractiveness
Key Questions
- How common is Inflammation and Pain Post-Cataract Surgery?
- What are the key findings of Inflammation and Pain Post-Cataract Surgery epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Inflammation and Pain Post-Cataract Surgery?
- What are the disease risks, burden, and unmet needs of Inflammation and Pain Post-Cataract Surgery?
- At what CAGR is the Inflammation and Pain Post-Cataract Surgery market and its epidemiology is expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Inflammation and Pain Post-Cataract Surgery market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Inflammation and Pain Post-Cataract Surgery in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2020–2034)?
- How many companies are currently developing therapies for the treatment of Inflammation and Pain Post-Cataract Surgery?
Frequently Asked Questions
1. Is Inflammation and pain post-cataract surgery hereditary?
No the inflammation and pain post-cataract surgery is not associated with any genetic factors.
2. Are there any treatments for inflammation and pain post-cataract surgery?
There are several approved therapies for inflammation and post-cataract surgery like DEXTENZA, and INVELTYS among others. Several off-label drugs are also recommended.
3. Are there any clinical trials for inflammation and pain post-cataract surgery treatments?
Research and clinical trials are ongoing to explore potential treatments for inflammation and pain post-cataract surgery. Some experimental treatments, such as VVN461 by VivaVision Biotech have shown promise in slowing disease progression.
4. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as incident cases, treatment costs, revenue generated, and market trends.
5. Is there an analysis of the market’s competitive landscape in the report?
The market forecast report will likely offer insights into key market players, their product offerings, partnerships, and strategies, providing stakeholders with a comprehensive understanding of the competitive dynamics in the inflammation and pain post-cataract surgery market.