Invasive Pneumococcal Disease Market
- The Invasive Pneumococcal Disease (IPD) market is projected to experience a rise in Compound Annual Growth Rate (CAGR) during the forecast period. Market revenue growth is largely fueled by the increasing burden of IPD, including hospitalizations, mortality, and significant healthcare costs, alongside the aging global population. This scenario is driving greater demand for both preventive and treatment options.
- The dynamics of the Invasive Pneumococcal Disease (IPD) market are anticipated to change in the coming years owing to the improvement in novel treatment options to treat IPD, increasing healthcare spending across the world. Key players in the treatment and prevention landscape are Pfizer, Merck, GSK, and others.
- Despite advancements in treatment options, several unmet needs persist in the management of invasive pneumococcal disease which include early diagnosis methods, evolving pneumococcal serotypes, antibiotic resistance and comprehensive patient education.
DelveInsight’s comprehensive report titled “Invasive Pneumococcal Disease Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Invasive Pneumococcal Disease (IPD). The report presents historical and projected epidemiological data covering Total Incident Cases of Invasive Pneumococcal Disease (IPD), Age-specific Incident Cases of Invasive Pneumococcal Disease (IPD), Gender-specific Incident Cases of Invasive Pneumococcal Disease (IPD), and Treatable Cases of Invasive Pneumococcal Disease (IPD), In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020-2034.
The report analyzes the existing treatment practices and unmet medical requirements in Invasive Pneumococcal Disease (IPD). It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
Invasive Pneumococcal Disease (IPD) Overview
Invasive pneumococcal disease is a well-known complication caused by infections with Streptococcus pneumoniae, which can be severe and frequently lead to fatalities. It predominantly affects young children and the elderly, with symptoms varying depending on the location of the infection and the health status of the host. Common clinical presentations of IPD include acute otitis media (middle ear infection), pneumonia, meningitis (infection of brain membranes), and bacteremia/sepsis, which can be life-threatening if untreated.
Several risk factors increase susceptibility to pneumococcal illness, including asplenia or splenic dysfunction, chronic respiratory disease, chronic heart disease, chronic kidney disease (including renal transplantation), chronic liver disease, and diabetes mellitus managed with insulin or medications. Additionally, individuals who are immunocompromised are at a higher risk.
Invasive Pneumococcal Disease (IPD) Diagnosis and Treatment Algorithm
Traditional diagnostic methods for IPD, such as culture and antigen detection, are slow or insufficiently sensitive. Molecular techniques like polymerase chain reaction (PCR) offer rapid and precise diagnosis from symptom onset. Isothermal methods like nicking endonuclease amplification reaction (NEAR), strand displacement amplification (SDA), loop-mediated isothermal amplification (LAMP), and helicase-dependent amplification (HAD) have introduced affordable and user-friendly diagnostic tools.
Vaccination stands as the foremost strategy for preventing invasive pneumococcal disease (IPD). Successful programs depend on pinpointing high-risk groups and understanding relevant risk factors. The two main vaccine types are Pneumococcal Conjugate Vaccine (PCV13) and Pneumococcal Polysaccharide Vaccine (PPSV23). PCV13 is expected to dominate due to its broader serotype coverage and superior immune response.
Treatment for IPD involves antimicrobial therapy tailored to the clinical syndrome, disease severity, patient age, comorbidities, recent antibiotic use, and local resistance patterns. Dexamethasone is frequently used as an adjunctive treatment for children over 6 Weeks old with suspected bacterial meningitis. Apart from the treatment options, pneumococcal vaccines have been developed to combat the disease burden, comprising polysaccharide antigens that coat bacterial surfaces and play a crucial role in pathogenicity.
According to the DelveInsight analysis, the incidence of IPD among hospitalized adults showed a slight upward trend in the early 2000s. However, during the early phase of the COVID-19 pandemic in 2020, there was a sudden decrease in pneumococcal disease incidence. Despite this, in 2022, the incidence gradually increased, returning to pre-pandemic levels. With the growing interest in prophylactic treatment we expect more positive outcomes especially in high-risk populations like children and the elderly in terms of disease burden and quality of life.
A notable concern in the treatment of IPD is the high percentage of cases that are not preventable by vaccines. According to the CDC, in 2021─2022, PCV20–13 and non-PCV serotypes accounted for 39.5% and 31.8% of cases respectively.
Invasive Pneumococcal Disease Epidemiology
The epidemiology section on the Invasive Pneumococcal Disease (IPD) market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the incidence of Invasive Pneumococcal Disease (IPD). Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
- From a survey conducted in Germany, the age-specific incidence of IPD is consistently higher in men than in women across all age groups, except for the youngest group (0-23 months), where the incidence is similar for both sexes.
- As per the insights, a significantly greater proportion of IPD rates were consistently higher among males than females in all age groups. The incidence of IPD is particularly elevated in older male adults, with the highest rates seen in men aged 65 years and above. Factors such as smoking, alcohol dependency, and specific chronic diseases that elevate the risk of IPD are more commonly found in the male population.
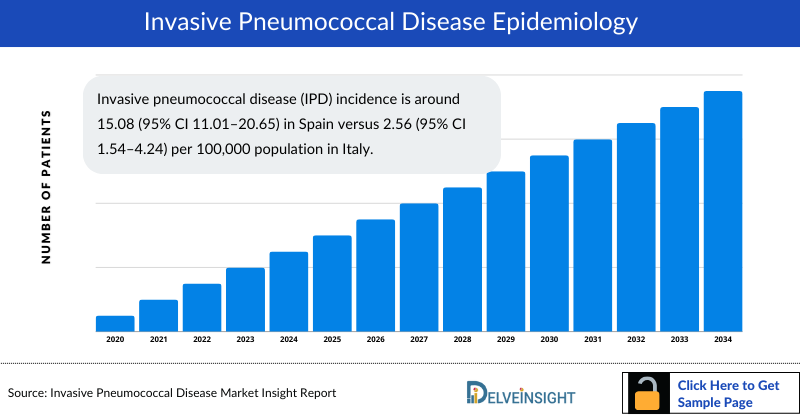
- Invasive pneumococcal disease (IPD) incidence is around 15.08 (95% CI 11.01–20.65) in Spain versus 2.56 (95% CI 1.54–4.24) per 100,000 population in Italy.
- As per the insights, IPD incidence in 2022–23 compared with 2019–20 was 34% higher in children (aged <15 years) and 17% lower in adults (aged 15 years and older).
- The annual incidence of IPD in children of <2 years of age was 145 cases per 100,000 individuals, compared with 72 and 32 per 100,000 individuals for children of <5 years of age and adults of >65 years of age, respectively.
- According to the European Centre for Disease Prevention and Control, the reported incidence of IPD in Europe varies from 0.4 to 20 cases per 100,000 population. However, surveillance strategies for IPD are heterogeneous across Europe making it difficult to compare data.
Invasive Pneumococcal Disease (IPD) Market Outlook
The management of Invasive Pneumococcal Disease (IPD) necessitates a comprehensive approach, combining various treatment strategies to effectively combat the infection and mitigate its impact. The primary treatment modalities include:
Antibiotic Therapy is the cornerstone of IPD treatment. Penicillin is often the first-line antibiotic, though its effectiveness can be hampered by antibiotic resistance. In such cases, cephalosporins like ceftriaxone and cefotaxime are commonly utilized, especially for severe infections. Macrolides, such as azithromycin and clarithromycin, provide an alternative for patients with penicillin allergies or as adjunct therapy. For highly resistant strains, vancomycin is a critical option, particularly in cases of meningitis caused by IPD.
Adjunctive Therapies play a crucial role in managing severe IPD. Corticosteroids are sometimes employed to reduce inflammation and prevent complications in severe cases like meningitis. Supportive care, including fluid management, pain control, and oxygen therapy, is essential to manage symptoms and support patient recovery.
Vaccination remains a pivotal preventive measure against IPD. The Pneumococcal Conjugate Vaccine (PCV13) protects against 13 types of pneumococcal bacteria and is recommended for children and high-risk adults. The Pneumococcal Polysaccharide Vaccine (PPSV23), covering 23 types of pneumococcal bacteria, is advised for adults over 65 and individuals with specific health conditions. These vaccines are instrumental in reducing the incidence of IPD and its associated complications.
CAPVAXIVE- In June 2024, CAPVAXIVE (V116) developed by Merck became the first Pneumococcal 21-valent Conjugate Vaccine to be approved for Prevention of Invasive Pneumococcal Disease and Pneumococcal Pneumonia in Adults. CAPVAXIVE is a potent vaccine which includes eight unique serotypes that are not covered by any other approved pneumococcal vaccines, accounting for about 27% of IPD cases in adults aged 50 and older and around 30% in those aged 65 and older, according to CDC data. Although the exact mechanism of action of the vaccine’s activity is unknown, CAPVAXIVE is designed to protect adults against the serotypes responsible for the majority of invasive pneumococcal disease (IPD) cases. The approval follows the FDA's Priority Review of Merck's application. Additionally CDC's Advisory Committee on Immunization Practices is set to meet later this month to discuss recommendations for CAPVAXIVE's use in adults.
Apart from this the PMDA approval of VAXNEUVANCE, a 15-valent conjugate vaccine manufactured by Merck Sharp & Dohme LLC also marks a milestone achievement in the treatment and management of IPD in Japan.
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the Invasive Pneumococcal Disease (IPD) market in the 7MM is projected to grow significantly during the study period 2020–2034.
Invasive Pneumococcal Disease (IPD) Drug Chapters
Marketed Invasive Pneumococcal Disease Drugs
VAXNEUVANCE: MSD K.K
VAXNEUVANCE, a 15-valent conjugate vaccine manufactured by MSD K.K. (Merck Sharp & Dohme LLC), is indicated for active immunization against invasive disease caused by specific Streptococcus pneumoniae serotypes in individuals aged six weeks and older. It is administered intramuscularly as a single dose and is contraindicated for those with a history of severe allergic reactions to any component of the vaccine or to diphtheria toxoid. The PMDA approved VAXNEUVANCE in 2023 as aqueous suspension syringes based on extensive Phase II and III studies in Japan, which demonstrated non-inferior immune responses for shared serotypes compared to PCV13, as well as superior responses for serotypes 3, 22F, and 33F. VAXNEUVANCE was initially approved by the US FDA in July 2021 for the prevention of IPD in adults and in 2022 for children and infants.
PREVENAR 20: Pfizer
The European Commission granted marketing authorization for PREVENAR 20, a 20-valent pneumococcal conjugate vaccine designed to prevent invasive disease, pneumonia, and acute otitis media caused by Streptococcus pneumoniae in infants, children, and adolescents aged six weeks to under 18. The FDA approved PREVENAR 20 in 2021 for adults and in 2023 for infants and children. This vaccine includes 13 serotypes already present in PREVENAR 13 and introduces seven new serotypes associated with high case-fatality rates and antibiotic resistance.
Emerging Invasive Pneumococcal Disease (IPD) Drugs
GSK5101956 (Pneumococcal 24-Valent – Adults) and (GSK5101955 Pneumococcal 24-Valent – Paed): GSK
GSK5101956 and GSK5101955 are in Phase II of clinical development for the prevention of pneumonia and invasive pneumococcal disease caused by the streptococcus pneumoniae 24 serotypes included in the vaccine, in adults aged 18 years and above and children aged 6 weeks - 17 years respectively. GSK5101956 and GSK5101955 are Multiple Antigen Presenting System (MAPS) platform vaccines. GSK5101956 has received Breakthrough Therapy Designation from the US FDA.
GBP-410: SK Bioscience/ Sanofi
GBP-410, also known as SP0202, is a next-generation pneumococcal conjugate vaccine developed by SK bioscience and Sanofi, who are co-investing in GBP410’s expansion. This candidate includes 21 serotypes, providing broader coverage than existing vaccines. Additionally positive Phase II trial results in June 2023 demonstrated comparable immunogenicity to PREVNAR 13. The companies are now preparing for a global Phase III trial, with regulatory submission anticipated in 2027. If successfully commercialized, GBP410 is projected to provide a 5─7% broader preventive range than the currently developed 20-valent vaccine for invasive pneumococcal disease (IPD) across all age groups.
Note: Detailed emerging therapies assessment will be provided in the final report...
Invasive Pneumococcal Disease (IPD) Market Segmentation
DelveInsight’s Invasive Pneumococcal Disease (IPD) Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Invasive Pneumococcal Disease (IPD) market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Invasive Pneumococcal Disease (IPD) Market Size by Countries
The Invasive Pneumococcal Disease (IPD) market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) Invasive Pneumococcal Disease (IPD) market, primarily attributed to the elevated cost of the available treatments and the increasing sensitivity toward pneumococcal infections. This dominance is projected to persist, especially with the potential early introduction of new products.
Invasive Pneumococcal Disease (IPD) Market Size by Therapies
Invasive Pneumococcal Disease (IPD) Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is GSK5101956 under the developmental pipeline of GSK.
Note: Detailed market segment assessment will be provided in the final report...
Invasive Pneumococcal Disease (IPD) Drugs Uptake
This section focuses on the sales uptake of potential Invasive Pneumococcal Disease (IPD) drugs that have recently been launched or are anticipated to be launched in the Invasive Pneumococcal Disease (IPD) market between 2020 and 2034. It estimates the market penetration of Invasive Pneumococcal Disease (IPD) drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Invasive Pneumococcal Disease (IPD) market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Invasive Pneumococcal Disease (IPD).
Invasive Pneumococcal Disease (IPD) Market Access and Reimbursement
DelveInsight’s ‘Invasive Pneumococcal Disease (IPD) – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Invasive Pneumococcal Disease (IPD).
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Invasive Pneumococcal Disease (IPD) market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Invasive Pneumococcal Disease (IPD) domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns for Invasive Pneumococcal Disease (IPD) market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market of Invasive Pneumococcal Disease (IPD) unmet needs.
Invasive Pneumococcal Disease (IPD): KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as University of Minnesota Children’s Hospital, Minneapolis, Minnesoto, National Institute of Infectious Diseases, Tokyo, Japan, NIHR Global Health Research Unit on Mucosal Pathogens, Division of Infection and Immunity, University College London, London, UK, Department of Biochemistry and Molecular Biology, Center for Molecular Medicine and Complex Carbohydrate Research Center, University of Georgia, Athens, GA, USA, Immunization, Hepatitis and Blood Safety Department, Health Protection Services, Colindale, Health Protection Agency, United Kingdom, Division of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA, and others.
“The seven-valent pneumococcal conjugate vaccine prevents invasive disease in both healthy and chronically ill children. The vaccine is effective when used with various non-standard schedules.”
Note: Detailed assessment of KOL Views will be provided in the full report on Invasive Pneumococcal Disease (IPD)...
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Invasive Pneumococcal Disease (IPD) Market, utilizing various Competitive Intelligence tools such as SWOT analysis, Conjoint Analysis, and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
The emerging Invasive Pneumococcal Disease (IPD) therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Invasive Pneumococcal Disease (IPD) market.
Invasive Pneumococcal Disease (IPD) Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for Invasive Pneumococcal Disease (IPD). It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Invasive Pneumococcal Disease (IPD).
Invasive Pneumococcal Disease Report Insights
- Invasive Pneumococcal Disease (IPD) Patient Population
- Invasive Pneumococcal Disease Therapeutic Approaches
- Invasive Pneumococcal Disease (IPD) Pipeline Analysis
- Invasive Pneumococcal Disease (IPD) Market Size and Trends
- Invasive Pneumococcal Disease (IPD) Market Opportunities
- Impact of Upcoming Invasive Pneumococcal Disease Therapies
Invasive Pneumococcal Disease (IPD) Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Invasive Pneumococcal Disease (IPD) Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Invasive Pneumococcal Disease (IPD) Market
- Invasive Pneumococcal Disease (IPD) Drugs Uptake
Invasive Pneumococcal Disease (IPD) Report Assessment
- Invasive Pneumococcal Disease (IPD) Current Treatment Practices
- Invasive Pneumococcal Disease Unmet Needs
- Invasive Pneumococcal Disease (IPD) Pipeline Product Profiles
- Invasive Pneumococcal Disease (IPD) Market Attractiveness
- Invasive Pneumococcal Disease Market Drivers
- Invasive Pneumococcal Disease Market Barriers
Key Questions
- How common is Invasive Pneumococcal Disease (IPD)?
- What are the key findings of Invasive Pneumococcal Disease (IPD) epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Invasive Pneumococcal Disease (IPD)?
- What is the disease risk, burden, and unmet needs of Invasive Pneumococcal Disease (IPD)?
- At what CAGR is the Invasive Pneumococcal Disease (IPD) market and its epidemiology expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Invasive Pneumococcal Disease (IPD) market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Invasive Pneumococcal Disease (IPD) in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2020–2034)?
- How many companies are currently developing therapies for the treatment Invasive Pneumococcal Disease (IPD)?
Frequently Asked Questions
1. What are the long term effects of Invasive Pneumococcal Disease (IPD) is left untreated?
If left untreated, invasive pneumococcal disease (IPD) can lead to serious long-term consequences, including significantly higher mortality rates compared to the general population, even among those who initially survive the acute infection. Vaccination is essential in preventing the severe outcomes associated with this disease.
2. Are there any treatments for Invasive Pneumococcal Disease (IPD)?
Yes, invasive pneumococcal diseases are treated with antibiotics. One of the best ways to help protect against certain types of infectious diseases, including pneumococcal, is through vaccinations like pneumococcal polysaccharide vaccine (PPV), etc.
3. Are there any clinical trials for Invasive Pneumococcal Disease (IPD) treatments?
Limited research and clinical trials are currently underway to investigate potential treatments for invasive pneumococcal disease (IPD). However, some experimental therapies have shown potential in slowing the progression of the disease.
4. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as incident cases, treatment costs, revenue generated, and market trends.
5. Is there an analysis of the market’s competitive landscape in the report?
The market forecast report will likely offer insights into key market players, their product offerings, partnerships, and strategies, providing stakeholders with a comprehensive understanding of the competitive dynamics in the Invasive Pneumococcal Disease (IPD) market