Liver cancer Market
Key Highlights
- According to Surveillance, Epidemiology, and End Results (SEER), in the US, the incidence rate of liver cancer and Intrahepatic bile duct cancer was 9.0 per 100,000 which is estimated to be around 42,230 newly diagnosed cases in 2021 and the mortality rate was 6.6 per 100,000 per year and the 5-year survival rate was 20.3%. Of them, the stage wise incident cases of liver cancer and intrahepatic bile duct cancer were Localized (45%), Regional (26%), Distant (18%), and Unknown (11%).
- Liver Cancer epidemiology is segmented as Total Incident Cases of Liver Cancer, Stage-wise Patients of Liver Cancer and Total Treated Cases of Liver Cancer] in the Liver Cancer market report.
Request for unlocking CAGR of the Liver Cancer Market
DelveInsight's "Liver Cancer Market Insights, Epidemiology, and Market Forecast — 2034" report delivers an in-depth understanding of the Liver Cancer, historical and forecasted epidemiology as well as the Liver Cancer market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The Liver Cancer market report provides current treatment practices, emerging drugs, Liver Cancer market share of the individual therapies, current and forecasted Liver Cancer market Size from 2020 to 2034 segmented by seven major markets. The Report also covers current Liver Cancer treatment practice/algorithm, and unmet medical needs to curate best of the opportunities and assesses the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, Spain, Italy, and France) and the United Kingdom
- Japan
Study Period: 2020–2034
Liver Cancer Market Disease Understanding and Treatment Algorithm
The DelveInsight’s Liver Cancer market report gives a thorough understanding of Liver Cancer by including details such as disease definition, symptoms, causes, pathophysiology, diagnosis, and treatment. Liver Cancer defined as a liver tumor not eligible for local therapies given the extent of disease or liver tumors that recurred after local therapies. Patients with advanced Hepatocellular Carcinoma (HCC) usually have significant underlying liver disease which is associated with poor tolerability to systemic chemotherapy.
The cancer may have spread to nearby lymph nodes and/or to distant sites within the body. Liver Cancer doesn't often metastasize, but when it does, it's most likely to spread to the lungs and bones. These cancers are widespread, they cannot be removed with surgery.
Signs and symptoms are not always directly related to the stage of the cancer, the effects of disease is highly individualized for each person and following are some of the symptoms occurred in individuals Gynecomastia, Erythrocytosis, High cholesterol, Hypercalcemia, Hypoglycemia.
Liver Cancer Diagnosis
Tests and procedures used to diagnose Liver Cancer include blood tests and liver tissue biopsy. Some of the tests and procedures used to diagnose liver cancer, such as CT scan and MRI, are also used in the staging process. A positron emission tomography (PET) scan may also be used: PET scan, which is a procedure used to find malignant tumor cells in the body.
Liver Cancer Treatment
Treatment decisions depend on the size of the cancer and whether it has spread. It also depends on the health of your liver tissue that is not affected by the cancer, for example if you have liver cirrhosis. Liver Cancer can now be treated with immunotherapy, some of the drugs, or treatment with a single drug include: atezolizumab and bevacizumab, lenvatinib, sorafenib.
Tecentriq is a drug that can increase the ability of the body's own immune system to target cancer. It might be used along with another drug, Avastin, which is a monoclonal antibody. These medications are given by infusion (intravenously) on a schedule that can be anywhere from two to four weeks. The use of high-energy particles is another potential treatment for stage 4 liver cancer. Two types that might be used are external beam radiation therapy (EBRT) and stereotactic body radiation therapy (SBRT).
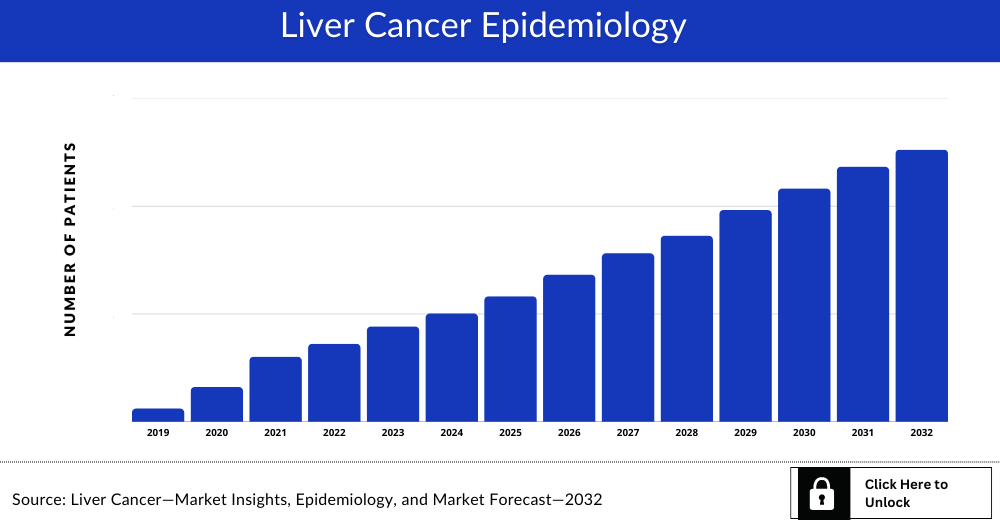
Liver Cancer Epidemiology
The Liver Cancer epidemiology section provides insights about historical and current Liver Cancer patient pool and forecasted trends for individual seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the report also provides the diagnosed patient pool and their trends along with assumptions undertaken.
Key Findings
- According to Surveillance, Epidemiology, and End Results (SEER), in the US, the incidence rate of liver cancer and Intrahepatic bile duct cancer was 9.0 per 100,000 which is estimated to be around 42,230 new diagnosed cases in 2021 and the mortality rate was 6.6 per 100,000 per year and the 5-year survival rate was 20.3%. Of them, the stage wise incident cases of liver cancer and intrahepatic bile duct cancer were Localized (45%), Regional (26%), Distant (18%), and Unknown (11%).
- The disease epidemiology covered in the report provides historical as well as forecasted Liver Cancer epidemiology [segmented as Total Incident Cases of Liver Cancer, Stage-wise Patients of Liver Cancer, and Total Treated Cases of Liver Cancer] in the 7MM covering the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan from 2020 to 2034.
Country Wise- Liver Cancer Epidemiology
This section provides a glimpse of Liver Cancer epidemiology in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Learn more about the evolving epidemiology trends and key developments: Liver Cancer Epidemiology Forecast
Liver Cancer Drug Chapters
The drug chapter segment of the Liver Cancer report encloses the detailed analysis of Liver Cancer marketed drugs and late stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Liver Cancer clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Liver Cancer Marketed Drugs
CYRAMZA (ramucirumabis): Eli Lilly
It is a VEGFR2 antagonist that specifically binds VEGFR2 and blocks binding of VEGFR ligands, VEGF-A, VEGF-C, and VEGF-D. As a result, ramucirumab inhibits ligand-stimulated activation of VEGFR2, thereby inhibiting ligand-induced proliferation, and migration of human endothelial cells.
KEYTRUDA (pembrolizumab): Merck
It is a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced liver cancer, including those with high microsatellite instability (MSI-H), DNA mismatch repair deficiency (dMMR), or high tumor mutational burden (TMB-H).
TECENTRIQ (atezolizumab): Genetech
It is a checkpoint inhibitor that targets the PD-L1 pathway; approved, in combination with bevacizumab, as a first-line treatment for subsets of patients with advanced liver cancer.
JEMPERLI (dostarlimab): GlaxoSmithKline
It is a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with Liver Cancerthat has DNA mismatch repair deficiency (dMMR).
OPDIVO (nivolumab): Bristol-Myers Squibb
It is a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced liver cancer, including in combination with ipilimumab.
YERVOY (ipilimumab): Bristol-Myers Squibb
It is a checkpoint inhibitor that targets the CTLA-4 pathway; approved, in combination with nivolumab, for patients with advanced, previously treated liver cancer.
Note: Detailed Current therapies assessment will be provided in the full report of Liver Cancer
Liver Cancer Emerging Drugs
H3B-6527, being developed by H3 Biomedicine, is a selective, orally bioavailable, and potent inhibitor of fibroblast growth factor receptor 4 (FGFR4) that is being investigated for the treatment of advanced hepatocellular carcinoma (HCC). The FDA’s Office of Orphan Drug Products grants orphan status to support development of medicines for rare diseases or conditions that affect fewer than 200,000 people in the U.S. The orphan drug designation provides H3 Biomedicine with certain benefits, including market exclusivity upon regulatory approval is received, exemption of FDA application fees, and tax credits for qualified clinical trials.
GNS561, being developed by Genoscience Pharma. is a first-in-class, orally bioavailable, small molecule that blocks cancer cell proliferation by inhibiting late-stage autophagy and dose-dependent build-up of enlarged lysosomes by interacting with the palmitoyl-protein thioesterase 1 (PPT1).
SAR445256 (known also as Alomfilimab, and formerly as KY1044) is a human monoclonal IgG1 that selectively binds to Inducible T cell CO-stimulator (ICOS), a protein expressed at high levels on immunosuppressive regulatory T cells and at lower levels on effector T cells. Being developed by Kymab in collaboration with Sanofi, it is designed to exert anti-tumour activity through preferential depletion of intra-tumoral regulatory T cells and stimulation (agonism) of ICOS-positive effector T cells.
Cabozantinib, being developed by Exelixis, is a targeted agent that inhibits the activity of receptor tyrosine kinases including MET, AXL, VEGF receptors and RET. These receptor tyrosine kinases are involved in both normal cellular function and in pathologic processes such as oncogenesis, metastasis, tumor angiogenesis, and resistance to multiple therapies, including immune checkpoint inhibitors (ICIs).
Note: Detailed emerging therapies assessment will be provided in the full report of Liver Cancer
Learn more about emerging therapies and key companies: Liver Cancer Pipeline Insight
Liver Cancer Market Outlook
There are currently six FDA-approved immunotherapy options for liver cancer. Several other immunotherapies are currently being tested in liver cancer clinical trial, including oncolytic viruses and adoptive cell therapy. Hence, the therapy market also include AVASTIN (bevacizumab) a targeted antibody that targets the VEGF-A pathway; approved, in combination with atezolizumab, as a first-line treatment for subsets of patients with advanced liver cancer.
Among patients who have successful surgeries, the rate of recurrence can be 50% or higher. In the current study, the researchers found that the five patients who underwent surgery and had their tumors shrink significantly have remained disease free for more than 230 days so far. Around 60% of patients who did not have a significant tumor response developed disease progression between 56 and 155 days after the end of treatment.
The dynamics of the Liver Cancer market is anticipated to change in the coming years owing to increased Research and Development activities and increased patient pool. Key players such as H3 Biomedicine, Genoscience Pharma, Kymab, Sanofi, Exelixis and various others are involved in developing therapies for Liver Cancer.
According to DelveInsight, Liver Cancer market in 7MM is expected to witness a major change in the study period 2020-2034.
Analyst Commentary
- The pipeline of Liver Cancer is very robust, many potential therapies are being investigated for the treatment of Liver Cancer, and it is safe to predict that the treatment space will experience a significant impact on the market during the forecast period.
- The expected introduction of emerging therapies with improved efficacy, more awareness initiatives programs, and further improvement in the diagnosis rate, are likely to boost the growth of the Liver Cancer market in the 7MM. Aside from that, the market size of Liver Cancer may flourish due to increased research and development, label-expansion of approved therapies into other epilepsy in this field.
- The market growth of Liver Cancer may be offset by failures and/or discontinuation of the emerging therapies, unaffordable pricing, market access and reimbursement issues, and a scarcity of healthcare specialists.
|
Report Metrics |
Details |
|
Study Period |
2020 to 2034 |
|
Base Year |
2024 |
|
Forecast Period |
2024 to 2034 |
|
CAGR | |
|
Liver cancer Drugs |
CYRAMZA (ramucirumabis), KEYTRUDA (pembrolizumab), TECENTRIQ (atezolizumab), JEMPERLI (dostarlimab), OPDIVO (nivolumab), YERVOY (ipilimumab), and Others. |
|
Key Companies |
Eli Lilly, Merck, Genetech, GlaxoSmithKline, Bristol-Myers Squibb, and Many Others. |
Liver Cancer Drugs Uptake
This section focuses on the rate of uptake of the potential drugs recently launched in the Liver Cancer market or expected to get launched in the market during the study period 2020-2034. The analysis covers Liver Cancer market uptake by drugs; patient uptake by therapies; and sales of each drug.
This helps in understanding the drugs with the most rapid uptake, reasons behind the maximal use of new drugs, and allows the comparison of the drugs based on market share and size which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Note: Detailed emerging therapies assessment will be provided in the full report of Liver Cancer.
Liver Cancer Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase II and Phase III stage. It also analyzes Liver Cancer key players involved in developing targeted therapeutics.
Liver Cancer Clinical Trial Development Activities
The Liver Cancer clinical trial report covers the detailed information of collaborations, acquisition and merger, licensing, patent details and other information for Liver Cancer emerging therapies.
Reimbursement Scenario in Liver Cancer
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In a report, we consider reimbursement to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
KOL- Views
To keep up with current market trends, we take KOLs and SMEs ' opinions working in the Liver Cancer domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies treatment patterns or Liver Cancer market trends. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the market and the unmet needs.
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Liver Cancer Market by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies etc. The inclusion of the analysis entirely depends upon the data availability.
Scope of the Liver Cancer Market Report
- The report covers the descriptive overview of Liver Cancer, explaining its causes, signs and symptoms, pathophysiology, diagnosis, and currently available therapies
- Comprehensive insight has been provided into the Liver Cancer epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging therapies for Liver Cancer are provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of the Liver Cancer market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- Detailed Patient Based Market Forecasting determines the trends shaping and driving the Global Liver Cancer market
Liver Cancer Market Report Highlights
- In the coming years, the Liver Cancer market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The companies and academics are working to assess challenges and seek opportunities that could influence Liver Cancer R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing therapies for Liver Cancer. The launch of emerging therapies will significantly impact the Liver Cancer market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Liver Cancer
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends, and comparative analysis of pipeline products with detailed liver cancer clinical trial profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Liver Cancer Report Insights
- Patient-Based Market Forecasting
- Therapeutic Approaches
- Liver Cancer Pipeline Analysis
- Liver Cancer Market Size and Trends
- Market Opportunities
- Impact of upcoming Therapies
Liver Cancer Report Key Strengths
- 11 Years Forecast
- 7MM Coverage
- Liver Cancer Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Market
- Drugs Uptake
Liver Cancer Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
Key Questions
Market Insights:
- What was the Liver Cancer drug class share (%) distribution in 2024 and how it would look like in 2034?
- What would be the Liver Cancer total market size as well as market size by therapies across the 7MM during the forecast period (2024-2034)?
- What are the key findings of the market across 7MM and which country will have the largest Liver Cancer market size during the forecast period (2024-2034)?
- At what CAGR, the Liver Cancer market is expected to grow by 7MM during the forecast period (2024-2034)?
- What would be the Liver Cancer market outlook across the 7MM during the forecast period (2024-2034)?
- What would be the Liver Cancer market growth till 2034, and what will be the resultant market Size in the year 2034?
- How would the unmet needs affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights:
- What are the disease risk, burden, and regional/ethnic differences of Liver Cancer?
- What are the key factors driving the epidemiology trend for seven major markets covering the United States, EU4 (Germany, Spain, Italy, and France) and the UK, and Japan?
- What is the historical Liver Cancer patient pool in seven major markets covering the United States, EU4 (Germany, Spain, Italy, and France) and the UK, and Japan?
- What would be the forecasted patient pool of Liver Cancer in seven major markets covering the United States, EU4 (Germany, Spain, Italy, and France) and the UK, and Japan?
- Where will be the growth opportunities in the 7MM concerning the patient population about Liver Cancer?
- Out of all 7MM countries, which country would have the highest incident population of Liver Cancer during the forecast period (2024-2034)?
- At what CAGR the patient population is expected to grow by 7MM during the forecast period (2024-2034)?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options for the Liver Cancer treatment in addition to the approved therapies?
- What are the current treatment guidelines for the treatment of Liver Cancer in the US, Europe, and Japan?
- What are the Liver Cancer marketed drugs and their respective MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many companies are developing therapies for the treatment of Liver Cancer?
- How many therapies are in-development by each company for Liver Cancer treatment?
- How many are emerging therapies in mid-stage, and late stage of development for Liver Cancer treatment?
- What are the key collaborations (Industry - Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Liver Cancer therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Liver Cancer and its status?
- What are the current challenges faced in drug development?
- What are the key designations that have been granted for the emerging therapies for Liver Cancer?
- What are the global historical and forecasted markets of Liver Cancer?
Reasons to buy
- The Patient Based Market Forecasting analysis will help in developing business strategies by understanding trends shaping and driving the Liver Cancer market
- Organize sales and marketing efforts by identifying the best opportunities for Liver Cancer in the US, Europe (Germany, Spain, Italy, and France) and the United Kingdom, and Japan
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for the Liver Cancer market
- To understand the future market competition in the Liver Cancer market