Lung Transplant Rejection Market
- The Lung Transplant Rejection market size is estimtaed to grow rapidly with a significant CAGR during the forecast period (2023-2032)
- Some of the key Lung Transplant Rejection companies developing therapies for Lung Transplant Rejection treatment are TFF Pharmaceuticals, Kamada, Corline Biomedical, MimeTech, Genentech, Bristol Myers Squibb, and others
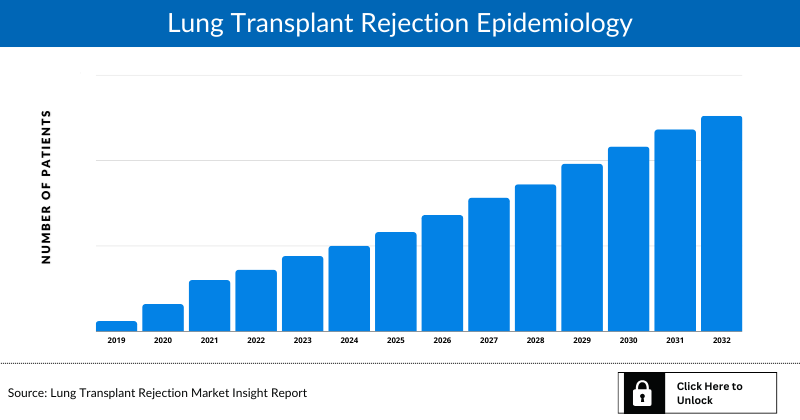
- The Lung Transplant Rejection epidemiology covered in the report provides historical as well as forecasted Lung Transplant Rejection epidemiology segmented as Total Prevalent Cases of Lung Transplant Rejection, Total Diagnosed Cases of Birch allergy, and Total Treated Cases of Lung Transplant Rejection in the 7MM covering the United States, EU4 (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2019 to 2032.
- The Lung Transplant Rejection epdemiology based on gender analzyed that Males comprise of 60% of the transplant recipients, where as females are around 40%. However, rejection statistics show no gender-based difference. Older individuals have a higher incidence of Lung transplant rejection than young individuals.
Request for Sample Page @ Lung Transplant Rejection Market Report
DelveInsight’s “Lung Transplant Rejection Market Insights, Epidemiology, and Market Forecast – 2032” report delivers an in-depth understanding of the Lung Transplant Rejection, historical and forecasted epidemiology as well as the Lung Transplant Rejection market trends in the United States, EU4 (Germany, France, Italy, Spain), and the United Kingdom, and Japan.
The Lung Transplant Rejection market report provides current treatment practices, emerging drugs, Lung Transplant Rejection market share of the individual therapies, and current and forecasted Lung Transplant Rejection market size from 2019 to 2032, segmented by seven major markets. The report also covers current Lung Transplant Rejection treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, Spain), and the United Kingdom
- Japan
Lung Transplant Rejection Treatment Market
The DelveInsight’s Lung Transplant Rejection market report gives a thorough understanding of Lung Transplant Rejection by including details such as disease definition, symptoms, causes, pathophysiology, diagnosis, and treatment.
Lung Transplant Rejection Overview
Lung transplantation is the definitive surgical treatment for select progressive end-stage lung disease patients despite being on optimum medical therapy. However, more than 30% of the LTx patients experience complications including rejection of transplanted lung.
Lung Transplant Rejection Diagnosis
During an evaluation, a doctor can confirm whether patients are experiencing lung rejection by performing a biopsy and looking for lymphocytes, a type of white blood cell that circulates in the blood and, in the case of rejection, attacks the blood vessels of the lungs. The severity of the rejection is ranked on a scale of zero to four, with four being the most severe.
Lung Transplant Rejection Treatment
The regimens which are employed to reduce the rate of lung transplant rejection vary from center to center, conventional maintenance therapy consists of triple drug therapy with a calcineurin inhibitor (cyclosporine or tacrolimus), antiproliferative agents [azathioprine (AZA), mycophenolate, sirolimus (srl), everolimus (evl)], and corticosteroids (CS). Roughly 50% of lung transplant utilizes induction therapy, with polyclonal antibody preparations [anti-thymocyte globulin (ATG)], interleukin 2 receptor antagonists (IL2RAs) (daclizumab or basiliximab), or alemtuzumabcauses should be treated with specific, directed antimicrobials.
Lung Transplant Rejection Epidemiology
The Lung Transplant Rejection epidemiology section provides insights into historical and current Lung Transplant Rejection patient pool and forecasted trends for seven individual major countries. It helps recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the Lung Transplant Rejection report also provides the diagnosed patient pool, their trends and assumptions undertaken.
Key findings
- In Sugimoto et al. (2023), mentioned in the study that the International Society for Heart and Lung Transplantation (ISHLT) reported, bilateral cadaveric LT (CLT) is performed in approximately 80% of patients worldwide. In contrast, compared with bilateral CLT (33.1%, 308 patients), the rate of single CLT (37.6%, 350 patients) and living-donor lobar lung (LDLLT) (29.0%, 270 patients) are relatively high in Japan because of the extreme donor shortage.
- According to Verleden and Gottlieb (2023), the number of LTxsworldwide is approximately 4,500 per year and bilateral LTx isperformed most frequently; however, unilateral LTx and combination transplant procedures can also be performed.
- Due to the fragility of the lung, the survival rates for lung transplant patients are not like other solid organ transplants, with a five-year survival rate of about 50-60%. And ~30% experiencing at least one rejection episode.
- According to the OPTN/SRTR annual report 2019, there were a total of 2,759 total lung transplants performed in the US. Among which 60% were male recipients and 40% were female recipients.
- The Registry of the International Society for Heart and Lung Transplantation reported that estimated chronic rejection between 1994 and 2014 was 50% and 67% within 5 and 10 years after transplantation, respectively.
- The Lung Transplant Rejection epidemiology covered in the report provides historical as well as forecasted Lung Transplant Rejection epidemiology [segmented as Total cases of Lung Transplantation, Type-specific cases of lung transplant rejection, Incident Cases of Lung Transplant Rejection, Treatable cases of lung transplant rejection] in the 7MM covering the United States, EU4 (Germany, France, Italy, Spain), and the United Kingdom, and Japan from 2019 to 2032.
Country-wise Lung Transplant Rejection Epidemiology
The epidemiology segment also provides the Lung Transplant Rejection epidemiology data and findings across the United States, EU4 (Germany, France, Italy, Spain), and the United Kingdom, and Japan.
Lung Transplant Rejection Drug Chapters
The drug chapter segment of the Lung Transplant Rejection report encloses the detailed analysis of Lung Transplant Rejection marketed drugs and late-stage (Phase III and Phase II) Lung Transplant Rejection pipeline drugs. It also helps understand the Lung Transplant Rejection clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Lung Transplant Rejection Marketed Drugs
Tacrolimus (Prograf): The US Food and Drug Administration (FDA) has approved the use of the transplant drug tacrolimus (Prograf) for the prevention of organ rejection in adult and pediatric patients receiving lung transplants. This is the only immunosuppressant drug approved for this patient population. Tacrolimus has been routinely prescribed to lung transplant recipients for the past 15 to 20 years and is the primary calcineurin inhibitor used as the backbone of immunosuppression for lung transplants.
Corticosteroids: Corticosteroids have been used in solid organ transplant. It is also used to treat acute cellular rejection (ACR) as well. The most commonly used corticosteroids in solid organ transplant are methylprednisolone and prednisone. Corticosteroids are known to have anti-inflammatory properties and exert their effects in a variety of ways, preventing T cell proliferation, decreasing macrophage activation, inhibiting cytokine production and altering lymphocyte migration. Initial doses range from 500-1,000 mg given intraoperatively, and are gradually tapered over weeks to months to 5-10 mg per day for maintenance.
Note: Detailed Current therapies assessment will be provided in the full report...
Emerging Lung Transplant Rejection Drugs
Belatacept- Bristol-Myers Squibb
Belatacept is a novel immunosuppressant approved for use in adult Lung transplantation, but has not been studied in lung transplantation. Belatacept is a selective costimulation blocker that binds CD80 and CD86, thereby blocking CD28-mediated costimulation in the T-cell activation cascade. It is currently in Phase II clinical trial for lung transplantation.
QXT-101– Qx Therapeutics
QXT-101 is a first in class treatment for patients who suffer from Acute Lung Injury (ALI). The mechanism of action (MOA) of QXT-101 involves the MAP3K2/3 pathway. The protein encoded by MAP3K2/3 gene is a member of serine/threonine protein kinase family. This kinase preferentially activates other kinases involved in the MAP kinase signaling pathway. It is currently being investigated in several indications including treatment of Primary Graft Dysfunction (PGD) in surgical lung transplant patients, ALI, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Note: Detailed emerging therapies assessment will be provided in the full report...
Lung Transplant Rejection Market Outlook
Lung transplantation can be a life-saving procedure for those with end-stage lung diseases. Unfortunately, long term graft and patient survival are limited by both acute and chronic allograft rejection, with a median survival of just over 6 years. The regimens which are employed to reduce the rate of Lung transplant rejection vary from center to center, conventional maintenance therapy consists of triple drug therapy with a calcineurin inhibitor (cyclosporine or tacrolimus), antiproliferative agents [azathioprine (AZA), mycophenolate, sirolimus (srl), everolimus (evl)], and corticosteroids (CS). Roughly 50% of lung transplant utilizes induction therapy, with polyclonal antibody preparations [anti-thymocyte globulin (ATG)], interleukin 2 receptor antagonists (IL2RAs) (daclizumab or basiliximab), or alemtuzumabcauses should be treated with specific, directed antimicrobials.
According to DelveInsight, the Lung Transplant Rejection market in 7MM is expected to witness a major change in the study period 2019–2032.
Key findings
This section includes a glimpse of the Lung Transplant Rejection market in 7MM.
The United States: Lung Transplant Rejection Market Outlook
This section provides the total Lung Transplant Rejection market size and market size by therapies in the United States.
EU4 and the UK Countries: Lung Transplant Rejection Market Outlook
The total Lung Transplant Rejection market size and market size by therapies in Germany, France, Italy, Spain, and the United Kingdom are provided in this section.
Japan: Lung Transplant Rejection Market Outlook
The total Lung Transplant Rejection market size and market size by therapies in Japan are also mentioned.
Lung Transplant Rejection Drug Uptake
This section focuses on the rate of uptake of the potential drugs recently launched in the Lung Transplant Rejection market or expected to get launched in the market during the study period 2019–2032. The analysis covers Lung Transplant Rejection market uptake by drugs, patient uptake by therapies, and sales of each drug.
This will help in understanding the Lung Transplant Rejection drugs with the most rapid uptake and the reasons behind the maximal use of new drugs and allows the comparison of the drugs based on market share and size, which again will be useful in investigating factors important in the market uptake and in making financial and regulatory decisions.
Lung Transplant Rejection Pipeline Development Activities
The Lung Transplant Rejection report provides insights into Lung Transplant Rejection Clinical Trails within Phase II, and Phase III stages. It also analyses Lung Transplant Rejection’s key players involved in developing targeted therapeutics.
Lung Transplant Rejection market clinical trial development activities
The report covers detailed information on collaborations, acquisitions, and mergers, licensing patent details, and other information for Lung Transplant Rejection emerging therapies.
Lung Transplant Rejection Reimbursement Scenario
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In a report, we consider reimbursement to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
KOL Views
To keep up with current epidemiology and market trends, we take KOLs and SMEs' opinions working in the Lung Transplant Rejection domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies and treatment patterns along with Lung Transplant Rejection market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Competitive Intelligence Analysis
We perform competitive and market intelligence analysis of the Lung Transplant Rejection market by using various competitive intelligence tools that include – SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
|
Report Metrics |
Details |
|
Study Period |
2019 to 2032 |
|
Forecast Period |
2023 to 2032 |
|
CAGR | |
|
Lung Transplant Rejection Market Size |
USD XX Million by 2032 |
|
Key Lung Transplant Rejection Companies |
Corline Biomedical, MimeTech, TFF Pharmaceuticals, Kamada, Genentech, Bristol Myers Squibb, Sanofi, Pfizer, and Many Others. |
Scope of the Lung Transplant Rejection Market Report
- Descriptive overview of Lung Transplant Rejection, disease overview, patient journeys, treatment algorithms, diagnosis, and currently available therapies
- Comprehensive insight into the Lung Transplant Rejection epidemiology and forecasts in the 7MM
- An all-inclusive account of both the current and emerging therapies for Lung Transplant Rejection, along with the assessment of new therapies, expected to have an impact on the current treatment landscape
- Exhaustive analysis of the Lung Transplant Rejection market; historical and forecasted covering drug outreach in the 7MM
- Detailed patient-based market forecasting determines the trends shaping and driving the global Lung Transplant Rejection market
Lung Transplant Rejection Market Report Highlights
- In the coming years, the Lung Transplant Rejection market is set to change due to the rising awareness of the disease and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The companies and academics are working to assess challenges and seek opportunities that could influence Lung Transplant Rejection R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing Lung Transplant Rejection therapies. The launch of emerging therapies will significantly impact the Lung Transplant Rejection market
- A better understanding of Lung Transplant Rejection pathogenesis will also contribute to the development of novel therapeutics for Lung Transplant Rejection
- Our in-depth analysis of the Lung Transplant Rejection pipeline assets across different stages of development (Phase III and Phase II), emerging trends, and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Lung Transplant Rejection Report Insights
- Lung Transplant Rejection Patient-Based Market Forecasting
- Lung Transplant Rejection Therapeutic approaches
- Lung Transplant Rejection pipeline analysis
- Lung Transplant Rejection market size and trends
- Lung Transplant Rejection market opportunities
- Impact of Upcoming Lung Transplant Rejection therapies
Lung Transplant Rejection Report Key Strengths
- 10 year forecast
- 7MM Coverage
- Lung Transplant Rejection epidemiology segmentation
- Key cross competition
- KOL views
- Lung Transplant Rejection drugs uptake
Lung Transplant Rejection Report Assessment
- Current Lung Transplant Rejection treatment practices
- Lung Transplant Rejection Unmet needs
- Lung Transplant Rejection pipeline product profiles
- Lung Transplant Rejection market attractiveness
- Lung Transplant Rejection Market Drivers
- Lung Transplant Rejection Market Barriers
Key Questions Lung Transplant Rejection Market Report
Lung Transplant Rejection market insights:
- What would be the Lung Transplant Rejection market growth till 2032, and what will be the resultant market size in 2032?
- What was the Lung Transplant Rejection drug class share (in percentage) distribution in 2019, and how would it look in 2032?
- What would be the Lung Transplant Rejection total market size and market size by therapies across the 7MM during the forecast period (2019–2032)?
- What are the key findings of the market across 7MM, and which country will have the largest Lung Transplant Rejection market size during the forecast period (2019–2032)
- How would the unmet needs affect the Lung Transplant Rejection market dynamics and subsequent analysis of the associated trends?
Lung Transplant Rejection Epidemiology Insights:
- What are the disease risk, burden, and regional/ethnic differences of Lung Transplant Rejection?
- What is the historical and forecasted Lung Transplant Rejection patient pool in 7MM, and where can one observe the highest patient population and growth opportunities?
- What are the key factors driving the epidemiology trends for seven major markets covering the United States, EU4 (Germany, France, Italy, Spain), and the UK, and Japan?
Current Lung Transplant Rejection Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current treatment guidelines and treatment options, in addition to approved therapies for Lung Transplant Rejection in the US, Europe, and Japan?
- What are the key collaborations (Industry–Industry, Industry-Academia), mergers and acquisitions, and licensing activities related to Lung Transplant Rejection therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Lung Transplant Rejection and its status, along with the challenges faced?
Reasons to Buy Lung Transplant Rejection Marekt Report
- The patient-based market forecast analysis will help in developing business strategies by understanding trends shaping and driving the Lung Transplant Rejection market
- Organize sales and marketing efforts by identifying the best opportunities for Lung Transplant Rejection in the US, EU4 (Germany, France, Italy, Spain), and the United Kingdom, and Japan
- Identification of strong upcoming players in the market that will help devise strategies that will help in getting ahead of competitors