Microscopic Polyangiitis Market Summary
- The Microscopic Polyangiitis market size in the 7MM was estimated to be more than USD 30 million in 2023 and is anticipated to grow with a significant CAGR during the study period (2020-2034).
- The Microscopic Polyangiitis Companies developing therapies include - Genentech, Inc. (F. Hoffmann-la roche ltd), Zenyaku Kogyo, Inflarx GMPH, Chemocentryx, Kissei Pharmaceutical, Vifor Pharma, Glaxosmithkline, Teijin Pharma, and others.
Microscopic Polyangiitis Market & Epidemiology Insights
- The commonly used immunosuppressive agents in microscopic polyangiitis management include cyclophosphamide, rituximab, methotrexate, glucocorticoids, azathioprine, and a few other biological agents. The cornerstone of microscopic polyangiitis treatment is corticosteroids, such as prednisone used in combination with other medications that suppress the immune system and reduce inflammation.
- The market is expected to grow by factors like the expected entry of emerging therapies with novel targets and pricing. Furthermore, the upcoming products such as Vilobelimab and Vynpenta are anticipated to expand the market with a deeper penetration in the 7MM.
- Amgen is the new owner of TAVNEOS (avacopan), an approved ANCA-associated vasculitis (AAV) therapy, following its successful USD 3.7 billion cash acquisition of ChemoCentryx.
- In September 2021, – VFMCRP announced that Japan’s Ministry of Health and Labor Welfare (MHLW) has granted its partner, Kissei Pharmaceutical, marketing authorization approval for TAVNEOS for the treatment of patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis.
- The US Food and Drug Administration approved RITUXAN (rituximab) injection to treat granulomatosis with polyangiitis and microscopic polyangiitis in children 2 years of age and older in combination with glucocorticoids.
- The approvals of biosimilars offer the potential for more treatment options and less expensive alternatives, which may pose a threat to novel drug makers. However, some of the critical issues affecting biosimilar uptake involve reimbursement factors and insurance coverage.
Request a sample to unlock the CAGR for "Microscopic Polyangiitis Market Forecast"
Key Factors Driving Microscopic Polyangiitis Market:
- Rising disease awareness and improved diagnosis: Enhanced recognition of vasculitis and availability of advanced diagnostic tools, including ANCA testing, are increasing identified cases of microscopic polyangiitis (MPA).
- Advancements in targeted therapies: Introduction of biologics, such as rituximab, and immunosuppressive agents are improving patient outcomes and expanding treatment options.
- High unmet medical need: Limited curative therapies and potential for severe organ damage drive demand for effective and long-term management solutions.
- Increasing prevalence of autoimmune disorders: A growing number of patients with autoimmune conditions contributes to the rising incidence of MPA.
- Supportive regulatory environment: Orphan drug designations and accelerated approval pathways encourage research and development of novel therapies for MPA.
DelveInsight’s "Microscopic Polyangiitis Market Insights, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of microscopic polyangiitis, historical and forecasted epidemiology as well as microscopic polyangiitis therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The microscopic polyangiitis market report provides current treatment practices, emerging drugs, microscopic polyangiitis market share of individual therapies, and current and forecasted microscopic polyangiitis market size from 2020 to 2034, segmented by seven major markets. The report also covers current microscopic polyangiitis treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Microscopic Polyangiitis Market |
|
|
Microscopic Polyangiitiss Market Size | |
|
Microscopic Polyangiitis Companies |
Genentech, Inc. (F. Hoffmann-la roche ltd), Zenyaku Kogyo, Inflarx GMPH, Chemocentryx, Kissei Pharmaceutical, Vifor Pharma, Glaxosmithkline, Teijin Pharma, and others. |
|
Microscopic Polyangiitis Epidemiology Segmentation |
|
Microscopic Polyangiitis Disease Understanding
Microscopic Polyangiitis Overview
Antineutrophil cytoplasmic autoantibody (ANCA) vasculitis is an autoimmune disease that causes swelling of the blood vessels. Several types of ANCA vasculitis exist due to numerous causes, so patients diagnosed with ANCA vasculitis may display varied symptoms. The ANCA-associated vasculitides (AAV) are three separate conditions, i.e., granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis (EGPA; previously known as Churg–Strauss syndrome [CSS]).
Microscopic polyangiitis is a rare autoimmune condition that causes blood vessel inflammation (vasculitis), restricting blood flow and lead to organ damage. The kidneys, lungs, nerves, skin, and joints are the most commonly affected areas of the body. The cause of is unknown, and it is not a contagious condition that does not usually show genetic predisposition and is not a form of cancer. The immune system is thought to play a critical role in the development of microscopic polyngiitis. It is believed that the immune system becomes overactive, but it still is not known that what causes this condition. microscopic polyngiitis symptoms depend on which blood vessels are involved and what organs in the body are affected. The most common symptoms of microscopic polyngiitis include kidney inflammation, weight loss, skin lesions, nerve damage, and fevers.
Microscopic Polyangiitis Diagnosis
Microscopic polyngiitis can be hard to diagnose, and the symptoms are similar to many other conditions. But, it is vital to receive diagnosis and treatment quickly to prevent permanent organ damage. The variable clinical manifestation of microscopic polyngiitis makes the diagnosis difficult. The diagnosis of microscopic polyngiitis is based on a combination of clinical, histopathological, and immunological criteria.
Microscopic polyngiitis diagnosis involves tests including complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein, urinalysis, serum creatinine, and tests for antineutrophil cytoplasmic antibodies (ANCA). ESR, C-reactive protein levels, and white blood cell and platelet counts are elevated, reflecting systemic inflammation.
Further details related to diagnosis will be provided in the report…
Microscopic Polyangiitis Treatment
Treatment of microscopic polyngiitis is based on several factors, including disease severity and organ involvement. microscopic polyngiitis management can be divided into four phases: identification, induction therapy, maintenance therapy, and long-term management. It is vital to know that remission does not imply the complete absence of symptoms; rather, it is used to convey the absence of symptoms attributable to active vasculitis.
The high levels of adverse effects with current therapies and their failure to induce full remission in all patients or prevent flare have driven a search for newer therapies. These have been immune-suppressive, pooled intravenous immunoglobulin or ‘biological’ agents, including therapeutic antibodies like Mycophenolate mofetil (MMF), Azathioprine, Methotrexate, Leflunomide, Rituximab, Etanercept.
Further details related to treatment will be provided in the report…..
Microscopic Polyangiitis Epidemiology
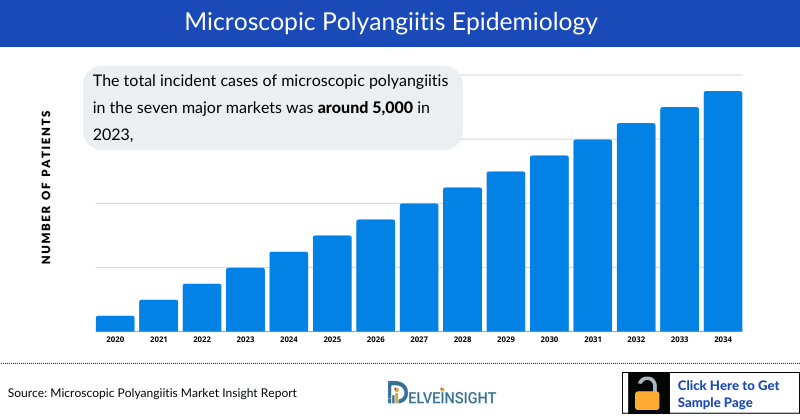
The Microscopic Polyangiitis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total incident cases of microscopic polyangiitis, gender-specific cases of microscopic polyangiitis , age-specific cases of microscopic polyangiitis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- The total incident cases of microscopic polyangiitis in the seven major markets was around 5,000 in 2023, which is expected to decrease in the forecast period. As the major contributor for the incident population are Japan and Germany where the population is declining, hence there is a decrease in the incidence is expected.
- In the US, the gender specific incident cases are dominated by males.
- The age‐specific data revealed that the highest number of microscopic polyngiitis incident cases are found in ≥18 years, while people in the age group 0–17 years are the least affected in the US.
Microscopic Polyangiitis Epidemiology Segmentation
- Total incident cases of Microscopic Polyangiitis
- Gender-specific cases of Microscopic Polyangiitis
- Age-specific cases of Microscopic Polyangiitis
Microscopic Polyangiitis Drug Analysis
The drug chapter segment of the Microscopic Polyangiitis report encloses a detailed analysis of the late-stage (Phase III ) and mid-stage (Phase II/III and Phase II) Microscopic Polyangiitis pipeline drugs. The current key players include InflaRx GmbH (Vilobelimab), GlaxoSmithKline (Belimumab) and others.
The drug chapter also helps understand the Microscopic Polyangiitis clinical trial details, pharmacological action, agreements and collaborations, approval, and patent details, and the latest news and press releases.
Microscopic Polyangiitis Marketed Drugs
RITUXAN: Genentech
The use of RITUXAN for the treatment of pediatric patients with GPA and microscopic polyngiitis 6 years of age and older is supported by evidence from adequate and well-controlled studies of RITUXAN in adults with GPA and microscopic polyngiitis; a trial in pediatric patients 6 years of age and older with active GPA and microscopic polyngiitis; and population pharmacokinetic (PK) analyses showing similar drug exposure levels in adults and pediatric patients 6 years to 17 years of age. The RITUXAN application for pediatric GPA and MPA was approved under a priority review, and with orphan designation, to fulfil an unmet medical need for these rare and serious diseases.
TAVNEOS: Vifor Pharma
TAVNEOS is a medicine used to treat adults with severe, active granulomatosis with polyangiitis (GPA or Wegener’s granulomatosis) or microscopic polyangiitis, which are inflammatory conditions of the blood vessels. TAVNEOS is used as part of a combined treatment also including the medicines rituximab or cyclophosphamide. TAVNEOS is the first approved treatment for people with severe active microscopic polyangiitis and granulomatosis with polyangiitis in over a decade, and marks the first FDA approval of an oral complement 5a receptor inhibitor for with ANCA-associated vasculitis.
Emerging Microscopic Polyangiitis Drugs
Vilobelimab (IFX-1, CaCP290): InflaRx GmbH
Vilobelimab is an intravenous, first-in-class monoclonal anti-human complement factor C5a antibody, which highly and effectively blocks the biological activity of C5a and demonstrates high selectivity toward its target in human blood. Thus, the drug leaves the formation of the membrane attack complex (C5b-9) intact as an important defense mechanism, which is not the case for molecules blocking the cleavage of C5.
Currently, Phase III enrollment is ongoing and the interim analysis for adaptation and fertility is anticipated in 2025.
Belimumab: GlaxoSmithKline
Belimumab is a medicine used as an add-on treatment in patients aged 5 years and older with systemic lupus erythematosus (SLE), a disease in which the immune system (the body’s natural defences) attacks normal cells and tissues, causing inflammation and organ damage. Belimumab is given to patients whose disease is still highly active despite standard treatment. Belimumab is also used in adults to treat active lupus nephritis, a manifestation of SLE causing kidney damage. In this case, it is given in combination with different immunosuppressants (medicines that reduce the activity of the immune system). Benlysta contains the active substance belimumab.
|
Product |
Company |
Phase |
MOA |
Molecule Type |
ROA |
|
Vilobelimab |
InflaRx GmbH |
III |
Attenuate inflammation and associated tissue damage by binding to complement factor 5a (C5a) |
Monoclonal Antibody |
Intravenous |
|
Belimumab |
GlaxoSmithKline |
II |
Works against the cytokine BLyS, also known as B-cell activating factor (BAFF) |
Monoclonal Antibody |
Oral |
Detailed emerging therapies assessment will be provided in the final report...
Microscopic Polyangiitis Market Outlook
Microscopic polyngiitis is a serious but treatable disease. Treatment is based on several factors, including disease severity and organ involvement. The aim of treatment has been defined in two parts: induction and subsequently maintenance of disease remission. It is important to know that remission does not imply the complete absence of symptoms; rather, it is used to convey the absence of symptoms attributable to active vasculitis. The commonly used immunosuppressive agents in microscopic polyngiitis management include cyclophosphamide, rituximab, methotrexate, glucocorticoids, azathioprine, and a few other biological agents. The cornerstone of microscopic polyngiitis treatment is corticosteroids, such as prednisone, used in combination with other medications that suppress the immune system and reduce inflammation.
Induction of remission for individuals with the non-severe disease is generally achieved using a combination of glucocorticoids and methotrexate or rituximab alone. For individuals with severe disease, induction using cyclophosphamide with glucocorticoids may be needed. The rituximab versus cyclophosphamide for induction of remission in the AAV trial (RAVE trial) established that comparable results could be achieved using rituximab, especially in individuals experiencing side effects of cyclophosphamide therapy. Typically remission is achieved in most individuals over 2–6 months.
Detailed market assessment will be provided in the final report.
Key Findings
- The market size of microscopic polyangiitis in the 7MM was estimated to be more than USD 30 million in 2023.
- According to the estimates, in 2023, the highest market size of microscopic polyangiitis was found in Japan, followed by the US.
Microscopic Polyangiitis Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034. The landscape of microscopic polyangiitis treatment has experienced a transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of oncologist, the professionals and the entire healthcare community in their tireless pursuit of advancing healthcare. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
The novel monoclonal anti-C5a antibody vilobelimab has also been studied in a phase II trial in patients. The results appear promising; in addition to decreased glucocorticoids toxicity index, a smaller number of treatment-emergent adverse events have been observed in vilobelimab-treated patients. However, the study was not powered statistically to compare the efficacy of vilobelimab to standard glucocorticoids treatment. This editorial summarizes the study findings and outlines potential future directions
Microscopic Polyangiitis Pipeline Development Activities
The report provides insights into Microscopic Polyangiitis clinical trials within Phase III, Phase II/III, and Phase II. It also analyzes key players involved in developing targeted therapeutics. Companies like InflaRx GmbH, GlaxoSmithKline, and others are actively engaged in late and mid-stage research and development efforts for microscopic polyangiitis The pipeline of microscopic polyangiitis possesses potential drugs. However, there is a positive outlook for the therapeutics market, with expectations of growth during the forecast period (2024–2034).
Microscopic Polyangiitis Pipeline Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for microscopic polyangiitis emerging therapy.
KOL Views on Microscopic Polyangiitis Market Report
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Microscopic Polyangiitis Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Analyst views. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Microscopic Polyangiitis Market Access and Reimbursement
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In the report, we consider reimbursement to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
Since the patients’ healthcare payments are substantial and such high healthcare expenditure is burdensome for the patients and their families. To help these patients, various third parties and nonprofits also have reimbursement schemes based on the demonstration of acceptable cost-effectiveness.
Detailed market access and reimbursement assessment will be provided in the final report....
Scope of the Microscopic Polyangiitis Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of microscopic polyangiitis, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the microscopic polyangiitis market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive microscopic polyangiitis.
Microscopic Polyangiitis Report Insights
- Microscopic Polyangiitis Patient Population
- Microscopic Polyangiitis Therapeutic Approaches
- Microscopic Polyangiitis Pipeline Analysis
- Microscopic Polyangiitis Market Size and Trends
- Existing and Future Market Opportunity
Microscopic Polyangiitis Report Key Strengths
- Eleven Years Forecast
- The 7MM Coverage
- Microscopic Polyangiitis Epidemiology Segmentation
- Key Cross Competition
- Microscopic Polyangiitis Drugs Uptake
- Key Microscopic Polyangiitis Market Forecast Assumptions
Microscopic Polyangiitis Report Assessment
- Current Microscopic Polyangiitis Treatment Practices
- Microscopic Polyangiitis Unmet Needs
- Microscopic Polyangiitis Pipeline Product Profiles
- Microscopic Polyangiitis Market Attractiveness
- Qualitative Analysis (SWOT and Analyst Views)
- Microscopic Polyangiitis Market Drivers
- Microscopic Polyangiitis Market Barriers
FAQs Regarding the Microscopic Polyangiitis Market Report:
- What was the microscopic polyangiitis market size, the market size by therapies, market share (%) distribution in 2023, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for microscopic polyangiitis ?
- What are the disease risks, burdens, and unmet needs of microscopic polyangiitis ? What will be the growth opportunities across the 7MM concerning the patient population with microscopic polyangiitis ?
- What are the current options for the treatment of microscopic polyangiitis? What are the current guidelines for treating microscopic polyangiitis in the 7MM?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What is the patient share in microscopic polyangiitis ?
Reasons to Buy Microscopic Polyangiitis Market Forecast Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving microscopic polyangiitis
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis ranking of class-wise potential current and emerging therapies under the analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.