Mycosis Fungoides Market
Key Highlights
- Mycosis fungoides, also known as Alibert-Bazin syndrome or granuloma fungoides, is the most common type of cutaneous T-cell lymphoma (CTCL). It is a cutaneous lymphoma that originates in the peripheral epidermotropic T-cells, specifically the memory T-cells (CD45RO+), which express the T-cell receptor (TCR) and CD4+ immunophenotype.
- It generally affects the skin, but may progress internally over time. Symptoms include rash, tumors, skin lesions, and itchy skin.
- The mycosis fungoides market is expected to grow steadily from 2025 to 2034, driven by rising awareness, earlier diagnosis, and established treatments, with targeted therapies, immunotherapies, and a growing research pipeline supporting continued expansion.
- POTELIGEO (mogamulizumab-kpkc) by Kyowa Kirin and ADCETRIS (brentuximab vedotin) by Pfizer are key targeted therapies for mycosis fungoides, offering disease-specific mechanisms that improve outcomes in relapsed or refractory cases.
- HYBRYTE (Soligenix) and Lacutamab (Innate Pharma) are emerging therapies for mycosis fungoides, with HYBRYTE utilizing topical photodynamic therapy via synthetic hypericin and Lacutamab employing a first-in-class anti-KIR3DL2 monoclonal antibody, both progressing through clinical development to address unmet needs in early-stage and relapsed/refractory disease.
- Innate Pharma presented results on Lacutamab in patients with relapsed and/or refractory mycosis fungoides, including long-term follow-up and translational data from the TELLOMAK Phase II trial, at the American Society of Clinical Oncology (ASCO) 2025 Annual Meeting.
Request for unlocking the CAGR of the Mycosis Fungoides Market
DelveInsight’s comprehensive report titled “Mycosis Fungoides — Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of mycosis fungoides. The report presents historical and projected epidemiological data covering incident cases of mycosis fungoides, age-specific cases of mycosis fungoides, gender-specific cases of mycosis fungoides, type-specific cases of mycosis fungoides and treated cases of mycosis fungoides. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing treatment practices and unmet medical requirements in mycosis fungoides. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Mycosis Fungoides Epidemiology |
|
|
Mycosis Fungoides Market |
|
|
Market Analysis |
|
|
Mycosis Fungoides Market players |
|
|
Future opportunity |
Future opportunities in mycosis fungoides build on established skin-directed and systemic therapies while focusing on targeted and immunotherapy approaches for advanced or refractory disease. Advances in molecular profiling are enabling more personalized, patient-centered care, supporting steady market growth. |
Key Factors Driving the Growth of the Mycosis Fungoides Market
Increasing Prevalence of Mycosis Fungoides
The mycosis fungoides market is expanding due to a rising number of mycosis fungoides cases, which is the most common type of cutaneous T-cell lymphoma. Studies have shown a steady increase in the incidence of this condition over time.
Advancements in Treatment Options
Significant progress in therapeutic options is a major catalyst for market growth. These advancements encompass a range of treatments, from innovative systemic therapies to improved skin-directed treatments.
Launch of Emerging Mycosis Fungoides Therapies
The anticipated launch of emerging therapies such as HYBRYTE (Soligenix), Lacutamab (Innate Pharma), Fenretinide (SciTech Development), PTX-100 (Prescient Therapeutics), and others will propel the mycosis fungoides market.
Mycosis Fungoides Overview
Mycosis fungoides is a form of cutaneous T-cell lymphoma that usually follows a slow, chronic course. It is characterized by the accumulation of lymphocytes in the skin, leading to the development of plaques and nodular lesions. In more advanced stages, ulcerating tumors may appear, and the malignant cells can spread to lymph nodes. The disease may also extend beyond the skin, affecting organs such as the liver, spleen, gastrointestinal tract, or central nervous system.
Mycosis fungoides progresses through three stages: patch, plaque, and tumor. The patch stage presents as flat, reddish (or yellowish in darker skin) areas, while the plaque stage features raised reddish-brown or greyish lesions. Both are considered early stages. In the tumor stage, large irregular lumps arise, developing from plaques or normal skin and affecting anybody area, including the face and head.
Mycosis Fungoides Diagnosis and Treatment Algorithm
Diagnosing mycosis fungoides requires clinicopathological correlation, often supported by dermoscopy to differentiate it from inflammatory dermatoses, erythroderma, or leprosy. Multiple skin biopsies are usually needed, particularly in early stages, with histology showing malignant CD4+ T-cell infiltrates.
Additional tests on biopsy samples may include immunohistochemistry, electron microscopy, in situ hybridization, PCR, FISH, and T-cell receptor studies. Blood tests, lymph node biopsy, and imaging (CT/PET) are performed when extracutaneous spread is suspected or for TNMB staging.
In early-stage mycosis fungoides (stage IIA or below), treatment typically involves skin-directed approaches such as topical corticosteroids, nitrogen mustard (mechlorethamine), bexarotene, imiquimod, PUVA, or UVB therapy. Localized radiation can be used for isolated lesions, while total skin electron beam therapy (TSEBT) may be recommended for widespread, symptomatic plaques, often in combination with systemic agents. If skin-directed therapies are insufficient, systemic treatments like retinoids, interferons, HDAC inhibitors, or low-dose methotrexate may be used, especially in patients with higher-risk disease features.
In advanced stages (IIB–IV), the disease is more variable and often relapsing. Treatment goals focus on symptom relief, disease control, and management of aggressive forms. Localized tumors may be treated with radiation, while generalized tumors or widespread disease may require TSEBT and systemic therapies. For refractory cases, allogeneic hematopoietic cell transplantation may be considered with curative intent.
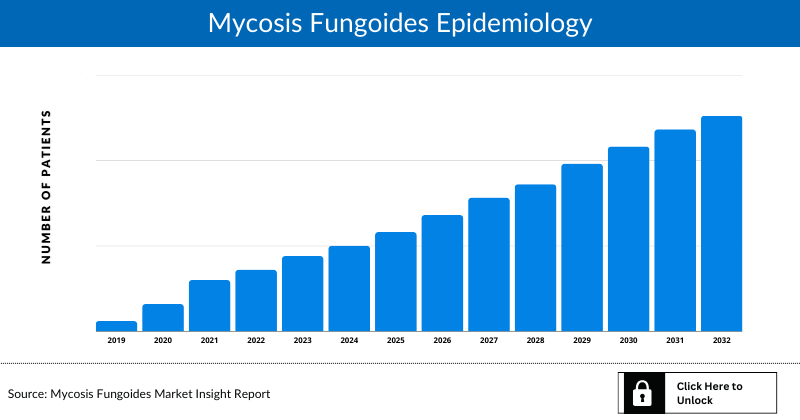
Mycosis Fungoides Epidemiology
The epidemiology section of the mycosis fungoides market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the incidence of mycosis fungoides. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
- According to secondary research, in Europe and the United States, mycosis fungoides occurs at a rate of about 6 cases per million people annually, representing roughly 4% of all non-Hodgkin lymphomas. It is seen more frequently in individuals over the age of 50.
- According to National Organization for the Rare Disorders (NORD), Mycosis fungoides is rare in individuals under 40 and occurs about twice as frequently in men as in women.
- The epidemiology of mycosis fungoides is expected to change during the forecast period (2025-2034).
Mycosis Fungoides Market Outlook
The mycosis fungoides therapeutics market is further expected to increase by the major drivers, such as the rising incidence population, technological advancements, and upcoming therapies in the forecast period (2025–2034).
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the mycosis fungoides in the 7MM is expected to change significantly during the forecast period 2025–2034.
Mycosis Fungoides Drug Chapters
Marketed Drugs
POTELIGEO (mogamulizumab-kpkc): Kyowa Kirin
POTELIGEO is a humanized monoclonal antibody (mAb) directed against CC chemokine receptor 4 (CCR4), which is frequently expressed on leukemic cells of certain blood cancers including CTCL. Using the proprietary POTELLIGENT technology, the amount of fucose in the sugar chain structure of POTELIGEO is reduced, which enhances the antibody dependent cellular cytotoxicity (ADCC). The US FDA granted Priority Review and Breakthrough Therapy Designation to POTELIGEO in 2017.
In August 2018, Kyowa Kirin announced that the US FDA had granted approval for POTELIGEO (mogamulizumab-kpkc) for the treatment of adult patients with relapsed or refractory mycosis fungoides or Sézary syndrome after at least one prior systemic therapy.
In November 2018, Kyowa Kirin announced that POTELIGEO had received marketing authorisation in Europe for the treatment of mycosis fungoides and Sézary syndrome.
ADCETRIS (brentuximab vedotin): Pfizer
ADCETRIS, a CD30-directed antibody–drug conjugate with microtubule inhibitory activity. FDA has granted Breakthrough Therapy Designation, Orphan Designation, and priority review to brentuximab vedotin for this indication.
In November 2017, the US FDA had granted regular approval to ADCETRIS (brentuximab vedotin) for the treatment of adult patients with primary cutaneous anaplastic large cell lymphoma (pcALCL) or CD30-expressing mycosis fungoides who had received prior systemic therapy.
|
Drug |
MoA |
RoA |
Company |
Logo |
|
POTELIGEO (mogamulizumab-kpkc) |
CCR4 inhibitor |
Intravenous |
Kyowa Kirin |
|
|
ADCETRIS (brentuximab vedotin) |
Targets CD30-expressing cells |
Intravenous |
Pfizer |
|
|
XX |
XX |
X |
XXX |
|
Note: Detailed marketed therapies assessment will be provided in the final report
Emerging Mycosis Fungoides Drugs
The mycosis fungoides market is expected to evolve gradually, driven by the limited number of emerging therapies currently in development. Key players such as HYBRYTE by Soligenix and Lacutamab by Innate Pharma among others are showing active commitment to addressing this unmet need, with ongoing efforts to advance novel treatment options for this complex condition.
HYBRYTE (Hypericin): Soligenix
HYBRYTE is a first-in-class photodynamic therapy for mycosis fungoides. It uses synthetic hypericin, a topical photosensitizer absorbed by malignant T-cells and activated by safe, visible red-yellow light after 24 hours. This light penetrates deeper than ultraviolet, enabling treatment of thicker lesions while avoiding the risk of UV-induced secondary cancers.
HYBRYTE has received orphan drug and fast track designations from the FDA, as well as orphan designation from the European Medicines Agency (EMA).
In April 2025, Soligenix announced interim results from an open-label investigator-initiated study of HYBRYTE (synthetic hypericin) in early-stage CTCL, showing 75% treatment success after 18 weeks, supporting its potential as a safe, fast-acting therapy for this chronic, underserved cancer. HYBRYTE is currently being evaluated in a Phase III clinical trial for mycosis fungoides.
Lacutamab (IPH4102): Innate Pharma
Lacutamab (IPH4102) is a first-in-class anti-KIR3DL2 humanized cytotoxicity-inducing antibody, which is currently in clinical trials for treatment of cutaneous T-cell lymphoma (CTCL), an orphan disease. This group of rare cutaneous lymphomas of T lymphocytes has a poor prognosis with few efficacious and safe therapeutic options at advanced stages.
Currently, the drug is in Phase II stage of its development for the treatment of mycosis fungoides.
Innate Pharma presented results on Lacutamab in patients with relapsed and/or refractory mycosis fungoides, including long-term follow-up and translational data from the TELLOMAK Phase II trial, at the American Society of Clinical Oncology (ASCO) 2025 Annual Meeting held May 30–June 3, 2025.
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
HYBRYTE (Hypericin) |
Photosensitizer |
Intravenous |
Soligenix |
|
III |
|
Lacutamab (IPH4102) |
KIR3DL2 receptor antagonists |
Intravenous |
Innate Pharma |
|
II |
|
XX |
XX |
X |
XX |
|
XX |
Note: Detailed emerging therapies assessment will be provided in the final report.
Mycosis Fungoides Market Segmentation
DelveInsight’s ‘Mycosis Fungoides – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future mycosis fungoides market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
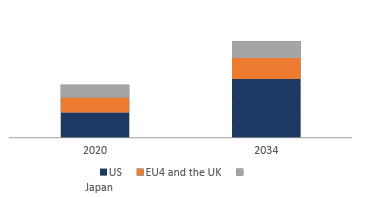
Mycosis Fungoides Market Size by Countries
The mycosis fungoides market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2024, the United States held a significant share of the overall 7MM (Seven Major Markets) mycosis fungoides market, primarily attributed to the country’s higher incidence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
|
Country-wise Market Size Distribution of Mycosis Fungoides |
|
|
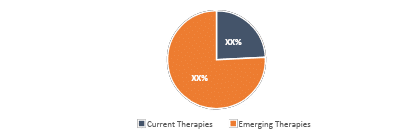
Mycosis Fungoides Market Size by Therapies
Mycosis Fungoides Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034.
|
Market Share Distribution of Mycosis Fungoides by Therapies in 2034 |
|
|
Note: Detailed market segment assessment will be provided in the final report.
Mycosis Fungoides Drugs Uptake
This section focuses on the sales uptake of potential mycosis fungoides drugs that have recently been launched or are anticipated to be launched in the mycosis fungoides market between 2025 and 2034. It estimates the market penetration of mycosis fungoides drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Mycosis Fungoides market.
The emerging mycosis fungoides therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Mycosis Fungoides market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Mycosis Fungoides.
Mycosis Fungoides Market Access and Reimbursement
DelveInsight’s ‘Mycosis Fungoides – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of mycosis fungoides.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current mycosis fungoides market trends and fill gaps in secondary findings, we interview KOLs and SMEs’ working in the mycosis fungoides domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or mycosis fungoides market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the mycosis fungoides unmet needs.
Mycosis Fungoides: KOL Insights
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as Columbia University, US, University of Freiburg, Germany, University of Bari, Italy, Université Paris-Saclay, France, University of Birmingham, UK, and Okayama University, Japan, among others.
“Classic mycosis fungoides is the most common subtype, followed by folliculotropic mycosis fungoides. Rare variants, such as pagetoid reticulosis and granulomatous slack skin, are extremely uncommon.”
“Given the heterogeneous and progressive characteristics of mycosis fungoides, treatment approaches are typically multimodal and tailored to the clinical stage, subtype, and site of disease involvement.”
“Maintenance therapy with agents like chlormethine gel is considered effective for managing persistent or resistant skin lesions in early-stage mycosis fungoides, offering a favorable balance between efficacy and tolerability.”
Note: Detailed assessment of KOL Views will be provided in the full report Mycosis Fungoides.
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the mycosis fungoides Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Mycosis Fungoides Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for mycosis fungoides. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging mycosis Fungoides therapies.
Mycosis Fungoides Report Insights
- Mycosis Fungoides Patient Population
- Therapeutic Approaches
- Mycosis Fungoides Pipeline Analysis
- Mycosis Fungoides Market Size and Trends
- Mycosis Fungoides Market Opportunities
- Impact of Upcoming Therapies
Mycosis Fungoides Report Key Strengths
- 10 Years Forecast
- The 7MM Coverage
- Mycosis Fungoides Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Mycosis Fungoides Market
- Mycosis Fungoides Drugs Uptake
Mycosis Fungoides Report Assessment
- Mycosis Fungoides Current Treatment Practices
- Unmet Needs
- Mycosis Fungoides Pipeline Product Profiles
- Mycosis Fungoides Market Attractiveness
Key Questions
- How common is mycosis fungoides?
- What are the key findings of mycosis fungoides epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for mycosis fungoides?
- What are the disease risk, burden, and unmet needs of mycosis fungoides?
- At what CAGR is the mycosis fungoides market and its epidemiology is expected to grow in the 7MM during the forecast period (2025–2034)?
- How would the unmet needs impact the mycosis fungoides market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of mycosis fungoides in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2025–2034)?
- How many companies are currently developing therapies for the treatment of mycosis fungoides?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the mycosis fungoides Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of current treatment in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Frequently Asked Questions
-
What are the treatment goals for Mycosis Fungoides?
- The primary treatment goals in mycosis fungoides are to achieve sustained disease control, alleviate symptoms, prevent progression to advanced stages, and preserve quality of life through a combination of skin-directed therapies, systemic treatments, and supportive care.
-
What are the challenges in managing Mycosis Fungoides?
- Management of mycosis fungoides is challenging due to its rarity, heterogeneous and often indolent skin manifestations, the potential for progression to advanced or extracutaneous disease, and the lack of curative treatment options, making long-term disease control and maintenance of quality of life difficult.
-
What are the key factors driving the growth of the Mycosis Fungoides market?
- Key factors driving growth in the mycosis fungoides market include rising awareness of the disease, improvements in early and accurate diagnosis, development and adoption of targeted and immunotherapeutic treatments, approval of new therapies, ongoing research and development investment, patient advocacy efforts, and supportive policies for rare diseases.
-
How will the Mycosis Fungoides Market and Epidemiology Forecast Report benefit the clients?
- The report will provide comprehensive insights into the current mycosis fungoides market landscape, emerging therapies, competitive dynamics, regulatory requirements, and market access considerations, enabling informed decision-making, strategic planning, and optimization of business strategies to capitalize on market opportunities and drive growth.
For More In-depth Information @ Latest DelveInsight Blogs





-and-sezary-syndrome-(ss)-pipeline.png&w=256&q=75)




