Non-metastatic Prostate Cancer (nmPC) Market
- Prostate cancer happens when abnormal cells form and grow in the prostate gland. Unlike benign (or non-cancerous) growths, these growths are cancerous (malignant). Prostate cancer can be life-threatening if it becomes “metastatic” and spreads beyond the prostate. Early stages of prostate cancer rely on testosterone to grow; sometimes, lowering testosterone can control growth.
- Approximately one in nine men in the US is diagnosed with this disease at some point. As per the American Cancer Society, 2019, prostate cancer is the second leading cause of cancer death in American men, behind only lung cancer.
- Castration-resistant prostate cancer (CRPC) is a form of advanced prostate cancer. If prostate cancer is diagnosed as CRPC, it can grow or spread even with low testosterone levels, and with non-metastatic CRPC (nmCRPC), the cancer no longer responds to hormone treatment. It shows signs of growth, like a rising PSA (prostate-specific antigen) level, but it is only found in the prostate and has not spread.
- Often, there are no symptoms for patients with nmCRPC. Most men cannot tell if the cancer is growing unless the doctor notices a rise in the PSA level. Changes are tracked with blood tests, physical exams, and scans, such as CT, bone, and PET scans.
- The main goal for treating nmCRPC is to shrink the tumor, control symptoms, and slow progress. The doctor may also suggest taking calcium and vitamin D to protect bones or other medications to help maintain bone density. The main treatments for nmCRPC include Androgen Deprivation Therapy (ADT), also called hormone therapy, second-line ADT, and active surveillance.
- In 2023, in the 7MM, the total initial therapy treated patient pool was maximum accounting, followed by biochemical recurrent/progressed treated patient pool.
- To date, only three drugs have been approved by the US FDA for nmCRPC, including XTANDI (Pfizer and Astellas), ERLEADA (Janssen Biotech), and NUBEQA (Bayer).
- Considerable success has been achieved in treating and managing prostate cancer; the challenge for scientists and clinicians to curve the speedy advancement of the disease still exists.
- In 2023, the total market size of non-metastatic prostate cancer in the 7MM was ~USD 5,300 million which is expected to reach ~USD 10,000 million by 2034.
Non- metastatic Prostate Cancer Market Report Summary
- The report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies and the elaborative profiles of late-stage (Phase III and Phase II) and prominent therapies that would impact the current treatment landscape and result in an overall market shift has been provided in the report.
- The report also encompasses a comprehensive analysis of the Non-metastatic Prostate Cancer market, providing an in-depth examination of its historical and projected market size (2020 – 2034). It also includes the market share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The report includes qualitative insights that provide an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM Non-metastatic Prostate Cancer market.
The table given below further depicts the key segments provided in the report:
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Epidemiology |
Segmented by:
|
|
Market |
Segmented by:
|
|
Market Analysis |
|
Non- metastatic Prostate Cancer Market
Various key players are leading the treatment landscape of Non-metastatic Prostate Cancer, such as Bayer, Janssen Pharmaceutical, Astellas Pharma, Pfizer, Myovant Sciences and others. The details of the country-wise and therapy-wise market size have been provided below.
- In EU4 and the UK, surgery captured the largest market share in 2023 among the first-line therapies i.e., ~USD 370 million.
- In 2023, the total market size of the United States was found to be ~USD 3,400 million which is expected to increase by 2034.
- Spain accounted for the least market size among the 7MM countries which was ~USD 160 million in 2023.
- The total market size of non-metastatic prostate cancer in Japan was ~USD 300 million in 2023, expected to reach ~USD 440 million by 2034.
Non- metastatic Prostate Cancer Drug Chapters
The section dedicated to drugs in the Non-metastatic Prostate Cancer report provides an in-depth evaluation of late-stage pipeline drugs (Phase III and Phase II) related to Non-metastatic Prostate Cancer.
The drug chapters section provides valuable information on various aspects related to clinical trials of Non-metastatic Prostate Cancer, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting Non-metastatic Prostate Cancer
Non- metastatic Prostate Cancer Marketed Therapies
ERLEADA (apalutamide): Janssen Pharmaceutical
ERLEADA (apalutamide) is a small molecule androgen receptor antagonist which binds to the intracellular receptor and prevents its translocation to the nucleus and subsequent DNA binding, thereby blocking its activity. Apalutamide is a third generation, oral nonsteroidal antiandrogen used to treat nonmetastatic castration-resistant prostate cancer. Therapy with apalutamide lowers residual testosterone levels after surgical castration in men with prostate cancer and has been shown to prolong metastasis free survival in men with castration-resistant prostate cancer with rising levels of prostate-associated antigen (PSA) without measurable metastatic disease.
XTANDI (enzalutamide): Astellas Pharma/Pfizer
XTANDI (enzalutamide) is an orally bioavailable, organic, non-steroidal small molecule targeting the AR with potential antineoplastic activity. Through a mechanism different from other approved AR antagonists, enzalutamide inhibits the activity of prostate cancer cell ARs, which may result in a reduction in prostate cancer cell proliferation and, correspondingly, a reduction in the serum PSA level. AR overexpression in prostate cancer represents a key mechanism associated with prostate cancer hormone resistance.
Emerging Non- metastatic Prostate Cancer Therapies
ORGOVYX (relugolix): Sumitomo Pharma/Pfizer
ORGOVYX (relugolix) is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist approved by the FDA for treating adult patients with advanced prostate cancer. As a GnRH antagonist, ORGOVYX blocks the GnRH receptor and reduces the production of testicular testosterone, a hormone known to stimulate the growth of prostate cancer. Relugolix is a non-peptide GnRH receptor antagonist that competitively binds to pituitary GnRH receptors, thereby reducing the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and, consequently, testosterone.
Eligible patients in clinical trial were treated with leuprolide acetate or degarelix with abiraterone or apalutamide prior to baseline, at which time they were transitioned to relugolix. Assessments included reporting of adverse events, clinical laboratory tests, vital sign measurements, electrocardiogram (ECG) parameters, and testosterone serum concentrations. The drug ORGOVYX is presently undergoing evaluation in a Phase I clinical trial to assess its safety and tolerability in patients.
CAN-2409: Candel Therapeutics
CAN-2409, Candel’s most advanced viral immunotherapy candidate, is a replication-defective adenovirus that delivers the herpes simplex virus thymidine kinase (HSV-tk) gene to cancer cells. HSV-tk is an enzyme that locally converts orally administered Val acyclovir into a toxic metabolite that kills nearby cancer cells. The intra-tumoral administration results in the release of tumor-specific neoantigens in the microenvironment. At the same time, the adenoviral serotype 5 capsid protein elicits a strong pro-inflammatory signal in the tumor microenvironment, which is designed to create the optimal conditions to induce an individualized and specific CD8+ T cell-mediated response against the injected tumor and uninjected distant metastases for broad anti-tumor.
The drug CAN-2409 is presently undergoing evaluation in a Phase I clinical trial to assess its safety and tolerability in patients.
|
Drug Name |
Company Name |
MoA |
Molecule Type |
Patient Segment |
Phase |
|
CAN-2409 + valacyclovir + radiation therapy ± ADT |
Candel Therapeutics |
Antigen unmasking and immune activation |
Non-replicative adenoviral vector |
Localized prostate cancer |
III |
|
ORGOVYX + ZYTIGA + corticosteroid + apalutamide/ docetaxel ± prednisone |
Myovant Sciences (Sumitomo Pharma)/ Pfizer |
GnRH receptor antagonist |
Small molecule |
nmCRPC |
I |
|
KPG-121 + enzalutamide, abiraterone/ apalutamide |
Kangpu Biopharmaceuticals |
CRBN E3 ubiquitin ligase/ CRL4 CRBN modulator |
Small molecules |
nmCRPC |
I |
Note: Detailed assessment will be provided in the final report of Non- metastatic Prostate Cancer…
Non- metastatic Prostate Cancer Market Outlook
The traditional therapeutic method for nmCRPC patients, androgen deprivation therapy (ADT) alone until metastatic disease, is considered inadequate. Several randomized Phase III clinical studies have shown considerable advantages for therapies combining ADT with apalutamide, enzalutamide, and darolutamide, including considerably improved overall survival (OS).
Many new molecules with novel mechanisms, like Androgen receptor inhibitor, Antigen unmasking and immune activation, GnRH receptor antagonist, CRBN E3 ubiquitin ligase/ CRL4 CRBN modulator among others, are being developed for the treatment of Non-metastatic prostate cancer by key players like Candel Therapeutics, Kangpu Biopharmaceuticals, Myovant Sciences, Janssen Pharmaceutical among others.
In conclusion, despite the lack of appropriate treatment in the current treatment landscape, many potential therapies with novel mechanisms are expected to enter the market, resolving a dire unmet need and leading to significant improvement in the treatment outcome of Non-metastatic Prostate Cancer patients. Hence, with the upcoming availability of new treatment options and increasing healthcare spending across the 7MM, the treatment scenario is expected to experience significant growth during the forecast period (2024–2034).
Further details are provided in the report...
Non- metastatic Prostate Cancer Understanding and Treatment
Overview
According to the Cancer Treatment Centers of America (CTCA), more than 99% of prostate cancers are adenocarcinomas, which develop in the gland cells. It is common in men 50–64 years and over 65; however, it can occur in men younger than 50. Symptoms of prostate adenocarcinoma include blood in the semen, frequent urge to urinate, and painful urination and ejaculation. The symptoms of prostate cancer do not usually appear until the prostate is large enough to affect the tube that carries urine from the bladder out of the penis.
Prostate cancer is one of the most common types of cancer found in men. This cancer usually grows slowly and is initially confined to the prostate gland, which may not cause serious harm. The exact cause of prostate cancer is unknown. However, several factors can increase the risk of developing this condition, like age, family history, diet, high testosterone level, and genome changes.
Further details are provided in the report…
Non- metastatic Prostate Cancer Diagnosis
Screening is testing to find cancer in people before they have symptoms. However, it is unclear if the benefits of prostate cancer screening outweigh the risks for most men. Still, after discussing the pros and cons of screening with their doctors, some men might reasonably choose to be screened. If one of these test results is abnormal, patients will probably need a prostate biopsy to know the cancer occurrence.
Many patients undergo regular prostate cancer screening before symptoms appear. Screening may involve one or more of the following tests: prostate-specific antigen (PSA), digital rectal exam (DRE), prostate ultrasound, prostate MRI, and prostate Mp-MRI.
Further details related to country-based variations are provided in the report...
Non- metastatic Prostate Cancer Treatment
The optimal management of localized prostate cancer starts with discussion by a multidisciplinary team (MDT) consisting of radiation oncologists, medical oncologists, urologists, histopathologists, radiologists, and specialist nurses. Each brings their expertise and knowledge of the patient to help determine which treatment options are appropriate for an individual.
The prostate cancer experts develop a comprehensive treatment plan for each patient specifically. Treatment strategies for prostate cancer depend on the stage and progression of cancer. However, prostate cancer often grows slowly, and active surveillance may be the preferred treatment option for some men, with the oncologist closely monitoring the disease with tests and holding off on treatment until a later date. Other treatment options for prostate cancers include chemotherapy, hormone therapy, immunotherapy, radiation therapy, and surgery.
Further details related to treatment and management are provided in the report...
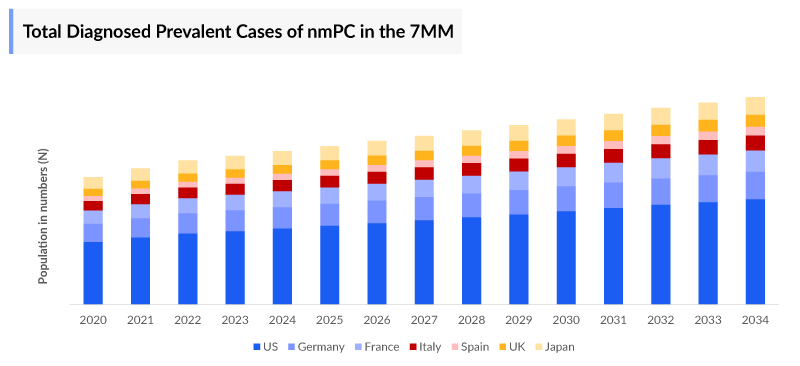
Non- metastatic Prostate Cancer Epidemiology
The Non-metastatic Prostate Cancer epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total Diagnosed Prevalent, Age-specific, total Stage-specific, total Line-wise Treated Cases of Prostate Cancer in the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
- Among EU4 countries, the highest number of diagnosed prevalent cases of non-metastatic prostate cancer was found in Germany with ~400,000 cases in 2023, followed by France and Italy.
- In 2023, among the age-specific cases, in the 7MM, highest cases were observed under the age group of > 84 years.
- In 2023, in the US, the total initial therapy treated patient pool was maximum accounting for ~699,000 cases, followed by biochemical recurrent/progressed treated patient pool i.e., ~190,000, and nmCRPC treated patient pool i.e., ~59,000.
Further details related to epidemiology will be provided in the report...
KOL Views
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of Non-metastatic Prostate Cancer, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 12 KOLs across the 7MM. We contacted institutions such as the Duke University School of Medicine, Samuel Oschin Cancer Center, Southwestern Medical Center, Cancer Institute of Montpellier, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Non-metastatic Prostate Cancer market, which will assist our clients in analyzing the overall epidemiology and market scenario.
Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in trials for Non-metastatic Prostate Cancer, important primary endpoints are overall survival rate, event-free survival, progression free survival, etc. Based on these parameters, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, a final weightage score is decided, based on which the emerging therapies are ranked.
Market Access and Reimbursement
Because newly authorized drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Non-metastatic Prostate Cancer Report Insights
- Non- metastatic Prostate Cancer Patient Population
- Non- metastatic Prostate Cancer Therapeutic Approaches
- Non-metastatic Prostate Cancer Market Size and Trends
- Existing Market Opportunity
Non-metastatic Prostate Cancer Report Key Strengths
- Ten-year Forecast
- The 7MM Coverage
- Non-metastatic Prostate Cancer Epidemiology Segmentation
- Key Cross Competition
Non-metastatic Prostate Cancer Report Assessment
- Current Non- metastatic Prostate Cancer Treatment Practices
- Non- metastatic Prostate Cancer Market Reimbursements
- Non- metastatic Prostate Cancer Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet needs)
- Non- metastatic Prostate Cancer Market Drivers
- Non- metastatic Prostate Cancer Market Barriers
Key Questions Answered In the Non- metastatic Prostate Cancer Market Report:
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Non-metastatic Prostate Cancer management recommendations?
- Would research and development advances pave the way for future tests and therapies for Non-metastatic Prostate Cancer?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of Non-metastatic Prostate Cancer?
- What kind of uptake will the new therapies witness in coming years in Non-metastatic Prostate Cancer patients?
-market.png&w=256&q=75)
.png)
.png)
-01.png)
.png)