Opioid Withdrawal Syndrome Market
- The total Opioid Withdrawal Syndrome Market Size in the 7MM was valued ~USD 1,302 million in 2022 and is anticipated to grow witha significant CAGR during the study period (2020-2034).
- DelveInsight’s analyst projects that the total number of Opioid withdrawal syndrome cases in 7MM were approximately 8,169,411 in 2022 and these cases are further expected to decrease during the forecasted period (2023-2034).
- Most number of opioid misuse cases were estimated in the US, followed by UK, Italy, and France in 2022.
- In the US, the market mainly consisted of opioid agonist-antagonist, Alpha-2 adrenergic agonists and others, which generated nearly USD 1,170 million in 2022.
- Leading Opioid Withdrawal Syndrome companies include Opiant Pharmaceuticals, Indivior, BioDelivery Sciences, Alkermes, and others developing innovative withdrawal management therapies.
- The total size of the Opioid withdrawal syndrome treatment market is anticipated to experience growth during the forecast period due to the emergence of new and effective treatments, namely DMX-1002 (Ibogaine), and others.
Request for Unlocking the Sample Page of the "Opioid Withdrawal Syndrome Treatment Market"
Key Factors Driving Opioid Withdrawal Syndrome Market
Growing Opioid Withdrawal Syndrome Patient Pool
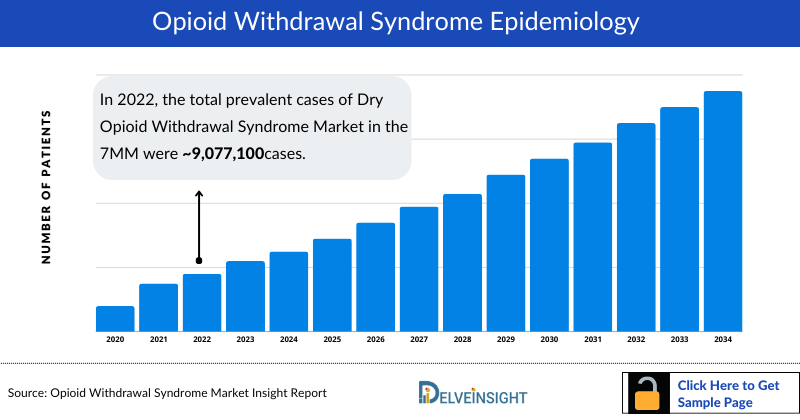
In 2022, DelveInsight estimated approximately 8.1 million diagnosed cases of opioid withdrawal syndrome across the 7MM. The United States recorded the highest burden, followed by the UK, Italy, and France. Additionally, around 9 million individuals were identified with long-term opioid usage, further fueling the patient pool. However, the overall number of withdrawal cases is projected to decline slightly during 2023–2034 due to preventive strategies and increasing treatment uptake.
Current Market Landscape
In the U.S., the opioid withdrawal syndrome market was valued at nearly USD 1.17 billion in 2022, mainly driven by opioid agonist-antagonists and alpha-2 adrenergic agonists. LUCEMYRA (lofexidine hydrochloride), marketed by US World Meds, is the only FDA-approved non-opioid therapy that helps reduce withdrawal symptoms for up to 14 days, though it is not a standalone treatment for opioid use disorder (OUD).
Emerging Therapies Transforming the Space
Promising therapies such as DMX-1002 (ibogaine; DemeRx/atai Life Sciences) and MN-166 (Medicinova) are in the pipeline, aiming to improve both withdrawal management and relapse prevention. DMX-1002, derived from the West African iboga plant, has shown potential as an acute detoxifier and is currently in phase I/II trials. MN-166, an oral small molecule glial attenuator with anti-inflammatory properties, is also being evaluated for its role in neuroinflammation and withdrawal management.
Expected Market Expansion
The opioid withdrawal syndrome market is anticipated to grow during the forecast period with the expected approval of DMX-1002, BXCL501, and other pipeline candidates. Among these, DMX-1002 is projected to capture the largest market share in the 7MM by 2034, given its dual potential in acute detoxification and long-term OUD treatment.
DelveInsight’s “Opioid Withdrawal Syndrome Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of the Opioid Withdrawal Syndrome, historical and forecasted epidemiology and the Opioid Withdrawal Syndrome therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Opioid Withdrawal Syndrome Treatment Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM, Opioid Withdrawal Syndrome market size from 2020 to 2034. The report also covers current Opioid Withdrawal Syndrome Treatment Market practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Opioid Withdrawal Syndrome Market |
|
|
Opioid Withdrawal Syndromes Market Size | |
|
Opioid Withdrawal Syndrome Companies |
DemeRx IB, Inc., atai Life Sciences, MediciNova, BioXcel Therapeutics Inc., and others. |
|
Opioid Withdrawal Syndrome Epidemiology Segmentation |
|
Opioid Withdrawal Syndrome Treatment Market
Opioid withdrawal is a constellation of symptoms that occurs after abrupt cessation, therapeutic discontinuation, or dosage reduction of opioids (ie, µ-receptor agonists), or after administration of an opioid antagonist (naltrexone or naloxone) or in some cases partial opioid agonist (buprenorphine) to a person who is physically dependent on opioids as a result of persistent, regular use. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria, signs and symptoms of opioid withdrawal include lacrimation or rhinorrhea, piloerection "goose flesh," myalgia, diarrhea, nausea/vomiting, pupillary dilation, photophobia, insomnia, autonomic hyperactivity (tachypnea, hyperreflexia, tachycardia, sweating, hypertension, hyperthermia), and yawning.
Opioid Withdrawal Syndrome Diagnosis
Although there is no diagnostic test for opioid withdrawal, urine toxicology must be checked to rule out withdrawal from any other drugs or combination of drugs. Urine toxicology is positive for most opioids such as morphine, heroin, codeine, oxycodone, and propoxyphene) for 12 to 36 hours after use. Methadone, buprenorphine, and LAAM (L-alpha-acetylmethadol) will not be detected in positive urine opiate tests, and they must be specifically tested. Urine toxicology for other drugs (marijuana, cocaine, benzodiazepine, and amphetamines) may also be commonly positive in opiate users. ECG, complete blood count (CBC), blood alcohol level, and basic metabolic panel (BMP) should also be done. COWS (Clinical Opioid Withdrawal Scale) assessment for opioid withdrawal is commonly used to determine the severity of opioid withdrawal.
Further details related to country-based variations are provided in the report
Opioid Withdrawal Syndrome Treatment
The primary treatment for opioid withdrawal syndrome involves a combination of medications and supportive care. Medications like methadone, buprenorphine, and naltrexone are commonly used to alleviate withdrawal symptoms and manage cravings. These medications work by either mimicking the effects of opioids to a lesser extent or blocking the opioid receptors. Additionally, supportive care may include counseling, behavioral therapies, and psychosocial support to address the psychological aspects of addiction. Symptomatic treatment in opioid withdrawal includes loperamide for diarrhea, promethazine for nausea/vomiting, and ibuprofen for myalgia. Clonidine can be given to reduce blood pressure. Opioid Withdrawal Syndrome clinical trials explore innovative therapies to manage symptoms, improve recovery outcomes, and support safer opioid dependence treatments.
Opioid Withdrawal Syndrome Epidemiology
As the market is derived using the patient-based model, the Opioid Withdrawal Syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Number of Opioid misuse cases, Number of cases with long-term opioid usage, and Total Opioid Withdrawal Syndrome Cases in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan, from 2020 to 2034.
- According to DelveInsight estimations, 9,077,124 Number of cases with long-term opioid usage were found in 2022 in the 7MM.
- As per DelveInsight’s estimations, the total number of cases with opioid withdrawal syndrome in the United States were approximately 7,087,561 in 2022 and are projected to decrease during the forecast period.
- According to DelveInsight’s estimates, the number of opioid misuse cases in EU4 and the UK were found to be approximately 1,365,293 in 2022.
Stay ahead with insights on Opioid Withdrawal Syndrome prevalence and patient population projections.
Opioid Withdrawal Syndrome Drug Chapters
The drug chapter segment of the Opioid Withdrawal Syndrome Treatment Market report encloses a detailed analysis of Opioid Withdrawal Syndrome marketed drugs and late-stage (Phase III and Phase II) Opioid Withdrawal Syndrome pipeline drugs. It also understands Opioid Withdrawal Syndrome clinical trials details, expressive pharmacological action, agreements and collaborations, approval, and patent details, advantages and disadvantages of each included drug, and the latest Opioid Withdrawal Syndrome news and press releases. The Opioid Withdrawal Syndrome drugs market is evolving with increasing awareness, innovative therapies, and rising demand for effective treatment options.
Opioid Withdrawal Syndrome Marketed Drugs
- LUCEMYRA (lofexidine hydrochloride): US World Meds LLC
LUCEMYRA is an oral, selective alpha 2-adrenergic receptor agonist that reduces the release of norepinephrine. LUCEMYRA is not an opioid drug. While LUCEMYRA may lessen the severity of withdrawal symptoms, it may not completely prevent them and is only approved for treatment for up to 14 days. LUCEMYRA is not a treatment for OUD but can be used as part of a broader, long-term treatment plan for managing OUD. LUCEMYRA is under license from Britannia Pharmaceuticals, Ltd. USWM, LLC is the exclusive licensee and distributor of LUCEMYRA in the United States and Its territories.
Note: Detailed Marketed therapies assessment will be provided in the final report of Opioid Withdrawal Syndrome...
Opioid Withdrawal Syndrome Emerging Drugs
- DMX-1002 (Ibogaine HCl): DemeRx IB, Inc. /atai Life Sciences
DMX-1002 is an oral formulation of ibogaine, an oneirogenic indole alkaloid with cholinergic, glutamatergic, and monoaminergic receptor modulatory activity. Ibogaine is a natural indole alkaloid derived from the West African iboga plant and has previously been marketed as a stimulant and antidepressant in France under the brand name Lambarène. Known for its oneirophrenic and hallucinogenic properties, uncontrolled data from hundreds of patients suggest that Ibogaine is effective as both an acute detoxifier and treatment for opioid addiction.At present, DMX-1002 is in Phase I/II of clinical development for the treatment of Opiate Withdrawal Syndrome. As per the DemeRx pipeline, the drug is being developed for opioid withdrawal management and relapse prevention, whereas as per atai Life Sciences pipeline, the drug is being developed for OUD treatment.
- MN-166: Medicinova
MN-166 is a first in class, orally bioavailable, small molecule glial attenuator that suppresses pro-inflammatory cytokines IL-1ß, TNF-a, and IL-6 and may upregulate the anti-inflammatory cytokine IL-10. It has additionally been shown to be a toll-like receptor 4 (TLR4) functional antagonist that may contribute to its attenuation of neuroinflammation. While considered a New Molecular Entity, or NME, in the United States and Europe, it involves redirection of an approved drug, ibudilast, which was first approved in Japan more than 20 years ago.
Stay ahead with key updates on Opioid Withdrawal Syndrome treatments. Access the 2025 pipeline report for exclusive insights!
Opioid Withdrawal Syndrome Market Outlook
The treatment approach for Opioid Withdrawal Syndrome depends on the severity of the symptoms experienced by the individual. In cases of mild withdrawal, patients are encouraged to prioritize hydration by consuming at least 2–3 L of water daily and may benefit from supplementation with vitamins B and C to support overall health. Typically, symptomatic treatment and supportive care suffice for managing mild symptoms, focusing on alleviating discomfort and ensuring the patient's well-being during this phase.
For individuals experiencing moderate to severe opioid withdrawal, pharmacotherapy becomes a crucial component of treatment. Medications such as clonidine, an alpha-2 adrenergic agonist, are commonly used to alleviate physical symptoms such as sweating, diarrhea, and abdominal cramps. However, it is important to monitor for potential side effects such as drowsiness and low blood pressure. Buprenorphine is often preferred for moderate to severe withdrawal due to its efficacy in symptom relief and craving reduction. Nevertheless, caution is warranted, especially in patients with respiratory issues or diabetes.
- The Opioid Withdrawal Syndrome Market is expected to experience positive growth with the approval of potential drugs like DMX-1002 (Ibogaine), BXCL501 and others.
- The total Opioid Withdrawal Syndrome Market Size in the 7MM was around USD 1,302 million in 2022. This is estimated to increase by 2034 at a significant CAGR.
- In the 7MM, most of the Opioid Withdrawal Syndrome Market Share was accommodated by Opioid agonist-antagonist generating nearly USD 1,107 million in 2022.
- Among the 7MM, the US captured the highest market in 2022, covering a total of 90% market, followed by the UK, which is anticipated to grow during the forecast period (2023–2034).
- In 2022, EU4 and the UK captured nearly 9% of the total market in the 7MM.
- Among the forecasted emerging therapies, DMX-1002 (Ibogaine) is expected to capture the highest market in the 7MM by 2034.
Opioid Withdrawal Syndrome Drugs Uptake
This section focuses on the uptake rate of potential Opioid Withdrawal Syndrome drugs expected to launch in the market during 2023–2034. For example, DMX-1002 (Ibogaine) in the US is expected to be launched by 2028.
Opioid Withdrawal Syndrome Pipeline Development Activities
The Opioid Withdrawal Syndrome Therapeutics Market report provides insights into different Opioid Withdrawal Syndrome clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes Opioid Withdrawal Syndrome Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Opioid Withdrawal Syndrome emerging therapies.
KOL Views
To keep up with current Opioid Withdrawal Syndrome market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on evolving Opioid Withdrawal Syndrome treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from Texas A and M University, College Station, TX, USA, Baylor College of Medicine, Houston, Texas, University of Southern California, USA, Bremen Institute of Drug Research, University of Bremen, Germany, Meiji Pharmaceutical University, Tokyo and others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or Opioid Withdrawal Syndrome Opioid Withdrawal Syndrome market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Opioid Withdrawal Syndrome unmet needs.
Opioid Withdrawal Syndrome Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Opioid Withdrawal Syndrome treatment market landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Opioid Withdrawal Syndrome Therapeutics Market Access and Reimbursement
The high cost of therapies for the Opioid Withdrawal Syndrome treatment is a major factor restraining the growth of the drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The Opioid Withdrawal Syndrome Treatment Market report further provides detailed insights on the country accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Opioid Withdrawal Syndrome Treatment Market Report Scope
- The Opioid Withdrawal Syndrome Treatment Market report covers a segment of key events, an executive summary, a descriptive overview, explaining its causes, signs, and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will affect the current treatment landscape.
- A detailed review of the Opioid Withdrawal Syndrome drugs market, historical and forecasted Opioid Withdrawal Syndrome market size, Opioid Withdrawal Syndrome drugs market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The Opioid Withdrawal Syndrome therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Opioid Withdrawal Syndrome drugs market.
Opioid Withdrawal Syndrome Therapeutics Market Report Insights
- Patient-based Opioid Withdrawal Syndrome Market Forecasting
- Opioid Withdrawal Syndrome Therapeutic Approaches
- Opioid Withdrawal Syndrome Pipeline Drugs Analysis
- Opioid Withdrawal Syndrome Market Size
- Opioid Withdrawal Syndrome Market Trends
- Opioid Withdrawal Syndrome Drugs Market
- Existing and Future Opioid Withdrawal Syndrome Drugs Market Opportunity
Opioid Withdrawal Syndrome Therapeutics Market Report Key Strengths
- 12 years Opioid Withdrawal Syndrome Market Forecasting
- The 7MM Coverage
- Opioid Withdrawal Syndrome Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Opioid Withdrawal Syndrome Drugs Uptake
- Key Opioid Withdrawal Syndrome Market Forecast Assumptions
Opioid Withdrawal Syndrome Therapeutics Market Report Assessment
- Current Opioid Withdrawal Syndrome Treatment Market Practices
- Opioid Withdrawal Syndrome Unmet Needs
- Opioid Withdrawal Syndrome Pipeline Product Profiles
- Opioid Withdrawal Syndrome Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Opioid Withdrawal Syndrome Market Drivers
- Opioid Withdrawal Syndrome Market Barriers
Key Questions Answered In The Opioid Withdrawal Syndrome Market Report:
Opioid Withdrawal Syndrome Therapeutics Market Insights
- What was the Opioid Withdrawal Syndrome market size, the market size by therapies, and Opioid Withdrawal Syndrome drugs market share (%) distribution in 2020, and how would it all look in 2034? What are the contributing factors for this growth?
- What unmet needs are associated with the current Opioid Withdrawal Syndrome treatment market?
- What are the patents of Opioid Withdrawal Syndrome Emerging Therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for off-label therapies?
- How would the Opioid Withdrawal Syndrome market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Opioid Withdrawal Syndrome Epidemiology Insights
- What are the disease risks, burdens, and Opioid Withdrawal Syndrome unmet needs? What will be the growth opportunities across the 7MM concerning the Opioid Withdrawal Syndrome patient population?
- What is the historical and forecasted Opioid Withdrawal Syndrome patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What factors are affecting the diagnosis of the indication?
Current Opioid Withdrawal Syndrome Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for treating Opioid Withdrawal Syndrome? What are the current guidelines for treating Opioid Withdrawal Syndrome in the US and Europe?
- How many Opioid Withdrawal Syndrome companies are developing therapies for treating it?
- How many Opioid Withdrawal Syndrome emerging therapies are in the mid-stage and late stage of development?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What are the key designations that have been granted for the Opioid Withdrawal Syndrome emerging therapies?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted Opioid Withdrawal Syndrome Market?
Reasons to Buy Opioid Withdrawal Syndrome Market Forecast Report
- The Opioid Withdrawal Syndrome market forecasting report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Opioid Withdrawal Syndrome Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing Opioid Withdrawal Syndrome market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies that will help get ahead of competitors.
- Detailed analysis, and ranking of class-wise potential current, and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Opioid Withdrawal Syndrome Companies around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the Opioid Withdrawal Syndrome unmet needs of the existing market so that the upcoming Opioid Withdrawal Syndrome Companies can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles