Osteosarcoma Market Summary
- The Osteosarcoma Market Size is anticipated to grow with a significant CAGR during the study period (2020-2034).
- Osteosarcoma is highly heterogeneous in its manifestation, which permits division into several subtypes according to the degree of differentiation, location within the bone, and histological variation.
- The key Osteosarcoma companies actively involved in the Neuroleptic Malignant Syndrome Treatment include - Takeda Pharmaceuticals, OS Therapies, Y-mAbs Therapeutics, AlaMab Therapeutics, CSPC Pharmaceutical, MedPacto, Hansoh Pharmaceutical, Takeda, Acrotech Biopharma, Bayer, Exelixis, Nektar Therapeutics, Eisai, GlaxoSmithKline/Novartis, Aadi Bioscience, Vaccinex, Inc., National Cancer Institute/Assaf-Harofeh Medical Center, Eleison Pharmaceuticals, Aurora Biopharma, BioAtla, Iovance Biotherapeutics, Isofol Medical AB, Bristol-Myers Squibb, Cellestia Biotech, and others.
Osteosarcoma Market and Epidemiology Analysis
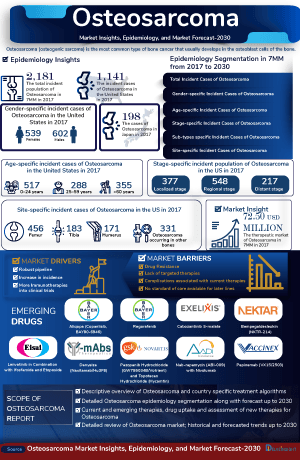
- Approximately 20–30% of patients with localized (non-metastatic) osteosarcoma and up to 80% of those with metastatic disease eventually develop relapsed or refractory osteosarcoma.
- Among all the subtype specific cases, chondroblastic accounted for ~5% of the total population share.
- In the US, the femur accounted for the highest proportion of site-specific incident cases, while tibia occupied the bottom of the ladder.
- Osteosarcoma is treated with a combination of therapies that can include surgery, chemotherapy, and radiation therapy. The mainstream chemotherapeutic agents for treatment include methotrexate, doxorubicin, cisplatin, and ifosfamide approved by the NCCN Guidelines.
- The preferred second-line (relapsed/refractory or metastatic) therapies by the NCCN include ifosfamide (high dose) and etoposide, regorafenib, and everolimus.
- FUSILEV (levoleucovorin) and KHAPZORY (levoleucovorin) was FDA-approved as “rescue” therapy after high-dose methotrexate treatment in osteosarcoma. FUSILEV was FDA approved in March 2008 and KHAPZORY was launched in 2019 as a lifecycle management extension of FUSILEV, just before generics entered the market. After patent expiration, FUSILEV was discontinued, but clinicians continue to use either KHAPZORY or generic levoleucovorin, as all products have the same indication for methotrexate rescue in osteosarcoma.
- MEPACT (mifamurtide) is an immunomodulatory therapy approved in Europe for children, adolescents, and young adults with high-grade, resectable, non-metastatic osteosarcoma after surgical removal of the tumor, where it is used alongside standard multi-agent chemotherapy to improve survival outcome.
- Recurrent osteosarcoma has a poor prognosis. Despite decades of research, new drugs and additional chemotherapy have brought little improvement, and there are still very few effective treatment options for relapsed patients.
- The emerging pipeline drug for osteosarcoma includes OST-HER2 (OS Therapies), Vactosertib (MedPacto), Surufatinib (Hutchmed), and others.
- OS Therapies plans to submit a Biologics License Application (BLA) to the US FDA for OST-HER2 in osteosarcoma by 2025. Upon approval, it may become eligible for a Priority Review Voucher, which could be sold for additional revenue.
Request a sample to unlock the CAGR for "Osteosarcoma Market Forecast"
Key Factors Driving The Osteosarcoma Market:
- Rising Incidence in Pediatric and Adolescent Populations: Osteosarcoma remains one of the most common primary bone cancers in children and young adults, sustaining long-term treatment demand.
- Growing Adoption of Combination Therapies: Increased use of multimodal treatment approaches such as chemotherapy, surgery, and targeted therapies is expanding the therapeutic landscape.
- Advances in Immunotherapy and Precision Medicine: Ongoing research into CAR-T cells, immune checkpoint inhibitors, and biomarker-based treatments is fueling innovation and clinical trial activity.
- Improved Diagnostic and Imaging Technologies: Enhanced MRI, PET-CT, and biopsy techniques allow earlier detection and better monitoring of tumor progression and metastasis.
- Rising Investments and Collaborations in Oncology R&D: Government grants, pharma-biotech partnerships, and orphan drug designations are accelerating drug development for this rare cancer.
DelveInsight's “Osteosarcoma Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of osteosarcoma epidemiology, market, and clinical development in osteosarcoma. In addition to this, the report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the osteosarcoma therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain ), the United Kingdom, and Japan.
Osteosarcoma Treatment Market Report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted osteosarcoma market size from 2020 to 2034 in 7MM. The report also covers current Kaposi sarcoma treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Osteosarcoma Market |
|
|
Osteosarcomas Market Size | |
|
Osteosarcoma Companies |
Takeda Pharmaceuticals, OS Therapies, Y-mAbs Therapeutics, AlaMab Therapeutics, CSPC Pharmaceutical, MedPacto, Hansoh Pharmaceutical, Takeda, Acrotech Biopharma, Bayer, Exelixis, Nektar Therapeutics, Eisai, GlaxoSmithKline/Novartis, Aadi Bioscience, Vaccinex, Inc., National Cancer Institute/Assaf-Harofeh Medical Center, Eleison Pharmaceuticals, Aurora Biopharma, BioAtla, Iovance Biotherapeutics, Isofol Medical AB, Bristol-Myers Squibb, Cellestia Biotech, and others. |
|
Osteosarcoma Epidemiology Segmentation |
|
Osteosarcoma Disease Understanding
Osteosarcoma Overview
Osteosarcoma, also known as osteogenic sarcoma is the most common type of bone cancer that originates from bone-forming cells that become cancerous and produce abnormal bone tissue. It usually develops in the long bones of the arms or legs, especially near the knee or shoulder. It predominantly affects the children, teenagers and young adults, especially when their bones are going rapidly. Further, individuals with osteosarcoma might feel of bone pain, swelling, swelling and fractures. It is caused due to complex genetic alterations, including mutations in tumour suppressor genes.
Osteosarcoma Diagnosis
The diagnosis of osteosarcoma involves clinical examination, imaging testing and biopsy. It begins with a physical exam, followed by imaging like X-rays, MRI, and CT scans to visualize the tumor and its extent. The definitive diagnosis is confirmed by biopsy, where a tissue sample is examined under a microscope to confirm cancer. This comprehensive approach ensures accurate diagnosis and helps in guiding treatment decisions.
Further details related to country-based variations in diagnosis are provided in the report...
Osteosarcoma Treatment
The treatment of osteosarcoma primarily involves the combination of surgery, chemotherapy and radiation therapy. Surgery aims to remove the tumor while preserving as much function and mobility as possible and may involve limb-sparing techniques or amputation. In some cases, rotationplasty may be performed, especially in children. The chemotherapy is given both before surgery (neoadjuvant) to shrink the tumor and after surgery (adjuvant) to eliminate any remaining cancer cells. The chemotherapy drugs include cisplatin, doxorubicin, ifosfamide, and high-dose methotrexate. Radiation therapy is less commonly used but may be considered when surgery is not possible or to relieve symptoms. Currently, MEPACT (mifamurtide) is the only approved for the treatment of osteosarcoma.
Further details related to treatment will be provided in the report…
Osteosarcoma Epidemiology
The Osteosarcoma epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total incident cases of osteosarcoma, site-specific cases of osteosarcoma, sub-types specific cases of osteosarcoma, stage-specific cases of osteosarcoma, age-specific cases of osteosarcoma, gender-specific cases of osteosarcoma in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
Key Findings in the Osteosarcoma Epidemiology Analysis
- Among the 7MM, the United States has captured the highest market share of osteosarcoma in 2024.
- Among all the subtype specific cases, around 80% were of conventional osteosarcoma in 2024.
- Osteosarcoma is more common in males as compared to females.
- Osteosarcoma is more common in children, teens, and young adults between the ages of 10 and 30, and only 1 in 10 osteosarcomas occur in people older than 60.
Osteosarcoma Epidemiology Segmentation
- Total Incident Cases of Osteosarcoma in the 7MM
- Site-specific Cases of Osteosarcoma in the 7MM
- Sub-types specific Cases of Osteosarcoma in the 7MM
- Stage-specific Cases of Osteosarcoma in the 7MM
- Age-specific Cases of Osteosarcoma in the 7MM
- Gender-specific Cases of Osteosarcoma in the 7MM
Osteosarcoma Market Recent Breakthroughs and Developments
- In September 2025, OS Therapies announced progress on its OST-HER2 program for recurrent pulmonary metastatic osteosarcoma after a productive FDA End of Phase 2 meeting, with plans to begin rolling a Biologics Licensing Application (BLA) submission that month.
- In January 2025, GSK’s B7-H3-targeted antibody-drug conjugate, GSK’227, received the Breakthrough Therapy Designation from US FDA in late-line relapsed or refractory osteosarcoma.
Osteosarcoma Drug Analysis
The drug chapter segment of osteosarcoma report encloses a detailed analysis of osteosarcoma marketed drugs and emerging pipeline drugs. It also deep dives into osteosarcoma’s pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Osteosarcoma Marketed Drugs
-
MEPACT (mifamurtide): Takeda
MEPACT is the first approved treatment for high-grade non-metastatic osteosarcoma after macroscopic surgery in patients aged between 2 to 30 years. It is used in combination with post-operative multi-agent chemotherapy.
In March 2009, the European Commission granted a marketing authorization valid throughout the European Union for MEPACT to IDM Pharm. Later in the same year, Takeda acquires IDM Pharma. Although, this product is not approved in the United States. Nevertheless, some osteosarcoma patients have been able to obtain MEPACT in the US through the FDA’s compassionate use and personal importation programs.
Note: Detailed current therapies assessment will be provided in the final report...
Osteosarcoma Emerging Drugs
-
DANYELZA (naxitamab 15-096): Y-mAbs Therapeutics
Naxitamab 15-096, a monoclonal antibody that targets GD2, was developed by investigators from Memorial Sloan Kettering Cancer Center (MSK) and is exclusively licensed by MSK to Y-mAbs Therapeutics. The drug is directed against the human tumor-associated antigen GD2, with potential antineoplastic activity. Upon vaccination, naxitamab stimulates antibody-dependent cell-mediated cytotoxicity (ADCC) against GD2-expressing tumor cells.
- In March 2025, the company announced the new data from a Phase II clinical trial evaluating naxitamab with granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with relapsed/refractory high-risk neuroblastoma and residual disease in the bone/bone marrow.
- In November 2024, Y-mAbs Therapeutics announced that they have entered into an exclusive license and distribution agreement for the development and commercialization in Japan of DANYELZA for the treatment of patients with relapsed/refractory high-risk neuroblastoma and, upon agreement by the parties, potentially relapsed osteosarcoma.
-
OST-HER2 (OST31-154): OS Therapies
OST-HER2, a biologic therapeutic candidate, is an Lm (Listeria monocytogenes) vector-based off-the-shelf immunotherapeutic vaccine designed to prevent metastasis, delay recurrence, and increase overall survival in patients with Osteosarcoma. The company announced the positive results for the Phase IIb AOST-2121 clinical trial in June 2024 and in October, 2024, the last patient was enrolled.
- The drug has been granted Orphan drug designation by the FDA and European Medicines Agency, Fast Track Designation by the FDA and Prime designation by EMA, and Rare Pediatric Disease Designation (RPDD) by FDA. Under the RPDD program, if the Company receives accelerated approval prior to September 30, 2026, it will become eligible to receive a Priority Review Voucher (PRV) that it intends to sell.
- In July 2025, OS Therapies granted end of Phase II Meeting by US FDA for OST-HER2 Program in the Prevention or Delay of Recurrent, Fully Resected, Pulmonary Metastatic Osteosarcoma. The Company expects the meeting to occur in the third quarter of 2025. The End of Phase II Meeting marks a pivotal point in the drug development process, and a significant milestone towards market access. The Company intends to seek alignment with FDA to begin a Rolling Review process for the forthcoming BLA submission for OST-HER2.
Table 1: Comparison of Emerging Drugs Under Development | ||||||
|
Drug Name |
Company |
Highest Phase |
Indication |
RoA |
MoA |
Molecule Type |
|
HS-20093 |
Hansoh Pharmaceutical |
II |
Patients with r/r osteosarcoma and other sarcomas |
IV Infusion |
B7-H3 Inhibitor |
Antibody Drug Conjugate (ADC) |
|
OST-HER2 |
OS Therapies |
IIb |
Patients with osteosarcoma |
IV Infusion |
Immune Stimulation |
tunable Antibody Drug Conjugate (tADC) |
|
Vactosertib |
MedPacto |
I/II |
Patients with recurrent, refractory, or progressive osteosarcoma |
Oral |
TGF-β receptor 1 Inhibitor |
Small Molecule |
|
ALMB-0168 |
AlaMab Therapeutics and CSPC Pharmaceutical |
I |
Patients with osteosarcoma |
IV Infusion |
Cx43 Inhibitor |
Monoclonal Antibody |
Note: Detailed emerging therapies assessment will be provided in the final report....
Osteosarcoma Drug Class Insights
This section covers various classes of therapies for osteosarcoma, including monoclonal antibodies, small molecules, antibody-drug conjugates, and immune checkpoint inhibitors.
Osteosarcoma Market Outlook
Treatment options typically involves surgery, chemotherapy, and radiation. While some new insights have emerged, major advancements have been limited since the survival gains seen in the late 1980s with chemotherapy. Radiation, which targets actively dividing cells, can cause early side effects like skin irritation and ulceration, and later effects such as hair loss, bone damage, fibrosis, and fractures.
Most patients with osteosarcoma in a limb can be treated with limb-sparing surgery, which removes the tumor while preserving the limb’s function and appearance. When this is not possible, amputation may be necessary, with patients often fitted with a prosthetic limb afterward. Rotationplasty is another surgical option involving removal of the tumor and knee joint, followed by reattachment of the lower leg to function as a new knee with a prosthesis. Chemotherapy, using drugs like cisplatin, doxorubicin, ifosfamide, and high-dose methotrexate with leucovorin rescue, improves overall survival by 60–70%. Despite this, about 70% of patients with localized disease respond successfully, while those with metastatic disease have a poorer prognosis, with only 20–30% achieving successful outcomes.
Further, the pipeline for osteosarcoma treatment, including ALMB-0168 (AlaMab Therapeutics in collaboration with CSPC Pharmaceutical), Naxitamab 15-096 (Y-mAbs Therapeutics), OST-HER2 (OST31-154, OS Therapies), and Vactosertib (MedPacto), has the potential to significantly transform the osteosarcoma treatment landscape and market dynamics in the coming years.
Osteosarcoma Drug Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034. The landscape of osteosarcoma treatment has experienced a profound transformation with the uptake of novel medicines. These innovative therapies are redefining standards of care.
Further detailed analysis of emerging therapies' drug uptake in the report...
Osteosarcoma Pipeline Development Activities
The Osteosarcoma Therapeutics Market Report provides insights into different Osteosarcoma clinical trials within the marketed and emerging stages. It also analyzes key Osteosarcoma Companies involved in developing targeted therapeutics. The Osteosarcoma clinical trials analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for osteosarcoma therapies.
KOL Views on the Osteosarcoma Report
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Professors, and Others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as UT Health San Antonio MD Anderson Cancer Center, The University of Arizona College of Medicine, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Osteosarcoma market trends.
|
KOL Views |
|
“Although osteosarcomas are chemo sensitive tumors, cancer cells can become drug resistant and have a tendency to form distant metastases. Using the tumor microenvironment as a potential therapeutic target indicates the start of a new era for osteosarcoma patients.”
|
Osteosarcoma Qualitative Analysis
We perform qualitative and market intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyses multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyse the effectiveness of therapy.
The analyst analyses multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry.
In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Osteosarcoma Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Osteosarcoma Market Report Scope
- The Osteosarcoma Treatment report covers a segment of key events, an executive summary, a descriptive overview of osteosarcoma, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the osteosarcoma market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Osteosarcoma Treatment Market Report provides an edge while developing business strategies, by understanding trends, through SWOT and conjoint analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM osteosarcoma market.
Osteosarcoma Market Report Insights
- Patient-based Osteosarcoma Market Forecasting
- Osteosarcoma Therapeutic Approaches
- Osteosarcoma Pipeline Analysis
- Osteosarcoma Market Size and Trends
- Existing and Future Osteosarcoma Drugs Market Opportunity
Osteosarcoma Market Report Key Strengths
- 10-Year Osteosarcoma Market Forecast
- 7MM Coverage
- Osteosarcoma Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Osteosarcoma Drugs Uptake
- Key Osteosarcoma Market Forecast Assumptions
Osteosarcoma Market Report Assessment
- Current Osteosarcoma Treatment Practices
- Osteosarcoma Unmet Needs
- Osteosarcoma Pipeline Drugs Profiles
- Osteosarcoma Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint)
- Osteosarcoma Market Drivers
- Osteosarcoma Market Barriers
Key Questions Answered in the Osteosarcoma Market Report
- What was the osteosarcoma market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors for this growth?
- At what CAGR, osteosarcoma market is expected to grow at the 7MM level during the study period (2020–2034)?
- How is Japan's osteosarcoma competitive landscape evolving?
- How will upcoming emerging therapies are going to impact MEPACT’s market share?
- What are the disease risks, burdens, and unmet needs of osteosarcoma?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to osteosarcoma?
- What is the historical and forecasted osteosarcoma patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What factors are affecting the increase in the diagnosis of symptomatic cases?
- What are the current options for the treatment of osteosarcoma? What are the current treatment guidelines for the treatment of Osteosarcoma in the US and Europe?
- How many companies are developing therapies for the treatment of osteosarcoma?
- Which key designations have been granted for the emerging therapies for osteosarcoma?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
Reasons to Buy the Osteosarcoma Market Forecast Report
- The Osteosarcoma Therapeutics Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics and driving factor for osteosarcoma market.
- Insights on patient share/disease burden, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Osteosarcoma Drugs Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying upcoming players in the Osteosarcoma Drugs Market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand KOLs’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Osteosarcoma Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for Recent Articles