Paroxysmal Supraventricular Tachycardia Market
Key Highlights
- Paroxysmal Supraventricular Tachycardia market is expected to experience steady growth during the forecast period (2024–2034). The increase in market size is a direct consequence of the increasing prevalent population of Paroxysmal Supraventricular Tachycardia patients in the 7MM.
- The Paroxysmal Supraventricular Tachycardia cases is expected to increase during the forecast period (2024–2034) due to an increase in valvular heart disease, heart failure, and diabetes mellitus among others.
- The Paroxysmal Supraventricular Tachycardia market is characterized by high competition, with numerous established companies such as Eisai and others, already offering treatments for Paroxysmal Supraventricular Tachycardia.
- To drive the Paroxysmal Supraventricular Tachycardia market in future years, several companies such as JIXING Pharmaceuticals/ Milestone Pharmaceuticals, and others are developing their assets in the mid-late stage of development. With the expected approval of these therapies during the forecast period [2024–2034], the overall therapeutic market of Paroxysmal Supraventricular Tachycardia is likely to witness a rise at a significant CAGR.
DelveInsight’s report titled “Paroxysmal Supraventricular Tachycardia – Market Insights, Epidemiology, and Market Forecast –2034” comprehensively analyzes Paroxysmal Supraventricular Tachycardia. The report includes a detailed examination of the historical and projected epidemiology data that includes total diagnosed prevalent cases of paroxysmal supraventricular tachycardia segmented by the gender and type. The market report on Paroxysmal Supraventricular Tachycardia provides comprehensive insights into different aspects concerning the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and future developments in the market across the seven major markets, namely the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
To gauge the market’s overall potential and identify business opportunities, the report discusses current Paroxysmal Supraventricular Tachycardia treatment practices and algorithms and unmet medical needs.
Paroxysmal Supraventricular Tachycardia Overview
Paroxysmal Supraventricular Tachycardia is a type of abnormal heartbeat rhythm that start and stops suddenly. It is caused by a rapid electrical signal that travels through the heart’s upper chambers (atria). This causes the heart to beat very fast, usually 160 to 220 beats per minutes.
There are several types of Paroxysmal Supraventricular Tachycardia, a type of abnormal heart rhythm. The most common types are atrioventricular nodal reentrant tachycardia (AVNRT) and atrioventricular reentrant tachycardia (AVRT).
Paroxysmal Supraventricular Tachycardia is the most frequent abnormal heart rhythm in newborns and infants. Wolff-Parkinson-White syndrome (WPW) is the most common type of Paroxysmal Supraventricular Tachycardia in children and infants. Paroxysmal Supraventricular Tachycardia is more common in adults under age 65. Adults over age 65 are more likely to have atrial fibrillation (AFib).
Episodes of Paroxysmal Supraventricular Tachycardia are often associated with symptoms including palpitations, sweating, chest pressure or pain, shortness of breath, sudden onset of fatigue, lightheadedness or dizziness, fainting, and anxiety.
Paroxysmal Supraventricular Tachycardia Diagnosis and Treatment Algorithm
Paroxysmal Supraventricular Tachycardia is a common tachyarrhythmia, and an electrocardiogram is the best tool for making a diagnosis. If Valsalva maneuvers and carotid sinus massage do not give positive results, then the next choice is either adenosine or calcium channel blockers. At this time, adenosine is the drug of choice for treatment. Verapamil and diltiazem are the most commonly used calcium channel blockers (CCBs).
The current treatment for Paroxysmal Supraventricular Tachycardia is a combination of drugs and procedures. Drugs are used to slow down the heart rate and prevent episodes of Paroxysmal Supraventricular Tachycardia. Procedures, such as catheter ablation, are used to destroy the tissue that is causing the arrhythmia.
Despite the availability of the effective treatment options, many people with Paroxysmal Supraventricular Tachycardia continue to experience symptoms. This is due to the severity of the arrhythmia, and the patient’s response to the treatment.
Paroxysmal Supraventricular Tachycardia Epidemiology
The Paroxysmal Supraventricular Tachycardia epidemiology section provides insights into the historical and current Paroxysmal Supraventricular Tachycardia patient pool and forecasted trends for seven individual major countries. It helps recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the report also provides the diagnosed patient pool, its trends, and assumptions undertaken.
The epidemiology section of the Paroxysmal Supraventricular Tachycardia market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
Key Findings
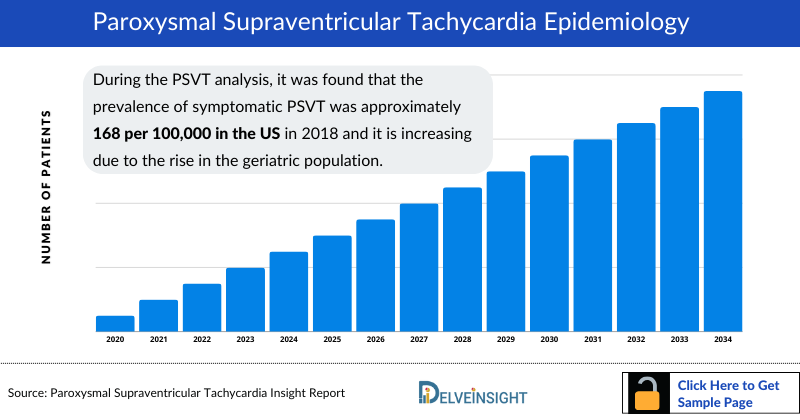
- During the Paroxysmal Supraventricular Tachycardia analysis, it was found that the prevalence of symptomatic Paroxysmal Supraventricular Tachycardia was approximately 168 per 100,000 in the US in 2018 and it is increasing due to the rise in the geriatric population. The Paroxysmal Supraventricular Tachycardia epidemiology is expected to change during the forecast period (2024–2034).
- The analysis indicates that the prevalence of Paroxysmal Supraventricular Tachycardia is higher in women. The prevalence of Paroxysmal Supraventricular Tachycardia is 1.3 – 1.6 times higher in women as compared to men.
- It is found that the most common type of Paroxysmal Supraventricular Tachycardia in adults is AVNRT which accounts for approximately 50 – 60% of the Paroxysmal Supraventricular Tachycardia population.
Paroxysmal Supraventricular Tachycardia Market Outlook
The goal of Paroxysmal Supraventricular Tachycardia treatment is to control the heart rate and prevent further episodes. With proper treatment, most people with Paroxysmal Supraventricular Tachycardia can live normal, active lives. There are a number of treatments available for Paroxysmal Supraventricular Tachycardia. The common treatments is to use a vagal maneuver, which is a technique that stimulates the vagus nerve, which slows down the heart rate. Other treatments include drugs (adenosine, beta-blocker, calcium channel blocker), catheter ablation and electrical cardioversion, which uses a shock to reset the heart’s rhythm. This evolving treatment landscape create opportunity for companies developing new and improved treatment for Paroxysmal Supraventricular Tachycardia.
Although there are existing treatment options, there remains a significant demand for more efficient and impactful treatments that can offer higher cure rates in a shorter duration. The expansion of the market for treating Paroxysmal Supraventricular Tachycardia is primarily propelled by advancements in medication and procedures. The development of novel medications aims to enhance effectiveness while minimizing adverse effects compared to older medications. Additionally, catheter ablation, a cutting-edge procedure, is increasingly accessible and utilized for a broader patient population.
Promising emerging treatment options are under investigation for managing Paroxysmal Supraventricular Tachycardia. Regulatory approvals, reimbursement policies, and access to these treatments will significantly shape the market dynamics. Additionally, increased awareness, early diagnosis may contribute to the identification of more individuals with Paroxysmal Supraventricular Tachycardia and create a demand for effective treatments.
According to DelveInsight, the Paroxysmal Supraventricular Tachycardia market in the 7MM is expected to change significantly during the study period 2020–2034.
Paroxysmal Supraventricular Tachycardia Drug Chapters
The drug chapter segment of the Paroxysmal Supraventricular Tachycardia report encloses the detailed analysis of Paroxysmal Supraventricular Tachycardia marketed drugs, mid-phase, and late-stage pipeline drugs. It also helps to understand the Paroxysmal Supraventricular Tachycardia clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details of each included drug, and the latest news and press releases.
Marketed Paroxysmal Supraventricular Tachycardia Drugs
VASOLAN: Eisai
VASOLAN is a calcium channel blocker with coronary/peripheral vasodilator actions. VASOLAN was approved in March 2008 for the treatment of Paroxysmal Supraventricular Tachycardia. It is the first oral form of calcium channel blockers approved for the treatment of tachyarrhythmia in Japan. VASOLAN intravenous injection was approved in May 2011 in Japan.
TAMBOCOR: Eisai
TAMBOCOR is a sodium channel blocker and is indicated for the treatment of Paroxysmal Supraventricular Tachycardia in pediatric patients. The effects of TAMBOCOR can be sustained stably with a twice-daily administration. TAMBOCOR was approved in May 2010 for the treatment of Paroxysmal Supraventricular Tachycardia in pediatric patients in Japan.
Emerging Paroxysmal Supraventricular Tachycardia Drugs
Paroxysmal Supraventricular Tachycardia market dynamics are anticipated to undergo transformation primarily driven by the global increase in healthcare expenditure. Paroxysmal Supraventricular Tachycardia Market players such as JIXING Pharmaceuticals/Milestone Pharmaceuticals, and others are actively involved in developing Paroxysmal Supraventricular Tachycardia treatments.
Etripamil: JIXING Pharmaceuticals/Milestone Pharmaceuticals
Etripamil, JIXING Pharmaceuticals lead investigational product, is a novel calcium channel blocker nasal spray. It is designed to be a rapid-response therapy that is self-administered by the patient, without the need for direct medical oversight, and is being developed for elevated and often highly symptomatic heart rate attacks associated with Paroxysmal Supraventricular Tachycardia and AFib-RVR. In May 2021, JIXING Pharmaceuticals entered into a exclusive license agreement with Milestone Pharmaceuticals to develop etripamil. Milestone is executing a comprehensive development program for etripamil, with Phase III studies completed and an NDA in Paroxysmal Supraventricular Tachycardia submitted to US FDA. Etripamil has the potential to be a valuable addition to the market for Paroxysmal Supraventricular Tachycardia treatment. It is a new, effective, and well-tolerated option for patients, and it can lead to increase the patient satisfaction, reduced healthcare costs, and improved quality of life.
In March 2024, Milestone announced the resubmission of its New Drug Application (NDA) to the US Food and Drug Administration (FDA) for etripamil, the Company’s lead investigational product for the management of Paroxysmal Supraventricular Tachycardia.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Etripamil |
calcium channel blocker |
Nasal Spray |
JIXING Pharmaceuticals/Milestone Pharmaceuticals |
III |
Note: Detailed emerging therapies assessment will be provided in the final report.
Paroxysmal Supraventricular Tachycardia Market Segmentation
DelveInsight’s ‘Paroxysmal Supraventricular Tachycardia – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Paroxysmal Supraventricular Tachycardia market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
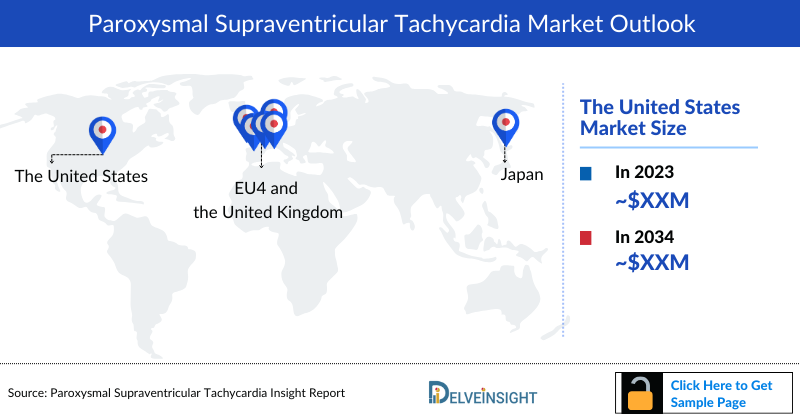
Paroxysmal Supraventricular Tachycardia Market Size by Countries
The Paroxysmal Supraventricular Tachycardia market size is analyzed for individual countries (the United States Market, EU4 [Germany, France, Italy, and Spain] and the UK market, and Japan). The United States accounted for a larger portion of the 7MM market for Paroxysmal Supraventricular Tachycardia in 2023 due to the high prevalence of the condition and the higher cost of treatments. This dominance is predicted to continue with the potential early entry of new products.
Paroxysmal Supraventricular Tachycardia Market Size by Therapies
Paroxysmal Supraventricular Tachycardia Market Size by Therapies is categorized into current market and emerging market for the study period 2020–2034. The emerging drug anticipated to launch during the forecast period includes Etripamil and others. As research and development efforts continue, it is expected that the market size for Paroxysmal Supraventricular Tachycardia therapies will expand further, driven by advancements in treatment options, increased awareness, and growing demand for innovative approaches to address the needs of individuals living with Paroxysmal Supraventricular Tachycardia.
Note: Detailed market segment assessment will be provided in the final report.
Paroxysmal Supraventricular Tachycardia Drugs Uptake
This section focuses on the sales uptake of potential Paroxysmal Supraventricular Tachycardia drugs that have recently launched or are anticipated to be launched in the Paroxysmal Supraventricular Tachycardia market between 2020 and 2034. It estimates the market penetration of the Paroxysmal Supraventricular Tachycardia drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Paroxysmal Supraventricular Tachycardia market.
The emerging Paroxysmal Supraventricular Tachycardia therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry, and other market dynamics, as well as the unmet need they fulfill in the Paroxysmal Supraventricular Tachycardia market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Paroxysmal Supraventricular Tachycardia.
Paroxysmal Supraventricular Tachycardia Market Access and Reimbursement
DelveInsight’s ‘Paroxysmal Supraventricular Tachycardia – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Paroxysmal Supraventricular Tachycardia.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Paroxysmal Supraventricular Tachycardia market trends and fulfill gaps in secondary findings, we interview KOLs and SMEs’ working in the Paroxysmal Supraventricular Tachycardia domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Paroxysmal Supraventricular Tachycardia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Paroxysmal Supraventricular Tachycardia unmet needs.
Paroxysmal Supraventricular Tachycardia: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Department of Cardiac Electrophysiology, Kaiser Permanente Santa Clara Medical Center, Santa Clara, CA., Department of Cardiology, Kaiser Permanente Oakland Medical Center, Oakland, CA, and others.
“Patients with Paroxysmal Supraventricular Tachycardia commonly experience multiple and unpredictable episodes throughout the year, which can greatly affect their quality of life.”
Note: Detailed assessment of KOL Views will be provided in the full report of Paroxysmal Supraventricular Tachycardia.
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Paroxysmal Supraventricular Tachycardia Market using various Competitive Intelligence tools, including SWOT analysis, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Paroxysmal Supraventricular Tachycardia Pipeline Development Activities
The report provides insights into therapeutic candidates in Phase II and III stages. It also analyses Paroxysmal Supraventricular Tachycardia companies involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Paroxysmal Supraventricular Tachycardia therapies.
Paroxysmal Supraventricular Tachycardia Report Insights
- Paroxysmal Supraventricular Tachycardia Patient Population
- Therapeutic Approaches
- Paroxysmal Supraventricular Tachycardia Pipeline Analysis
- Paroxysmal Supraventricular Tachycardia Market Size and Trends
- Paroxysmal Supraventricular Tachycardia Market Opportunities
- Impact of Upcoming Therapies
Paroxysmal Supraventricular Tachycardia Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Paroxysmal Supraventricular Tachycardia Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Paroxysmal Supraventricular Tachycardia Market
- Paroxysmal Supraventricular Tachycardia Drugs Uptake
Paroxysmal Supraventricular Tachycardia Report Assessment
- Paroxysmal Supraventricular Tachycardia Current Treatment Practices
- Unmet Needs
- Paroxysmal Supraventricular Tachycardia Pipeline Product Profiles
- Paroxysmal Supraventricular Tachycardia Market Attractiveness
Key Questions
- What are the key findings of the market across the 7MM, and which country will have the largest Paroxysmal Supraventricular Tachycardia market size during the forecast period (2024–2034)?
- At what CAGR is the Paroxysmal Supraventricular Tachycardia market, and epidemiology expected to grow in the 7MM during the forecast period (2024–2034)?
- In what ways would the unmet needs impact the Paroxysmal Supraventricular Tachycardia market dynamics and subsequently influence the analysis of related trends?
- What would be the forecasted patient pool of Paroxysmal Supraventricular Tachycardia in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan?
- What are the latest advancements in novel therapies, targets, mechanisms of action, and technologies being developed to address the limitations of existing therapies for Paroxysmal Supraventricular Tachycardia?
- How many companies are currently engaged in the development of therapies for the treatment of Paroxysmal Supraventricular Tachycardia?