PARP Inhibitors Market
Key Highlights:
- First runner LYNPARZA drew attention by treating patients with specific genetic mutations. LYNPARZA generated approximately USD 2.7 billion in revenue globally with approximately 40% revenue from the United States due to growth in usage in breast ovarian and prostate cancers.
- In contrast, latecomer ZEJULA rapidly expands market share by confirming its effects in all patients regardless of genetic mutations and proving “all-comer” indications.
- PARP inhibitors, including Olaparib, Niraparib, Rucaparib, and Talazoparib, have received approval as standalone treatments from regulatory bodies such as the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). Currently, PARP inhibitors are used for the treatment of various types of cancer, including breast cancer, ovarian cancer, fallopian tube cancer, primary peritoneal cancer, prostate cancer, and pancreatic cancer.
- Moreover, several PARP inhibitors are currently being evaluated in clinical trials. An example is AbbVie's Veliparib, which is in the developmental stage and is anticipated to receive approval during the forecast period.
- With up to 85% of patients experiencing disease recurrence that requires additional treatment, olaparib, and niraparib have played an important role in reducing the risk of recurrence since their approvals as maintenance therapies.
- PARPi possesses the unique ability to improve progression-free survival and repair DNA damage in cells. Attributed to this, these inhibitors are extensively used for the treatment of various types of cancers. Hence, the increasing burden of cancer is estimated to create lucrative sales opportunities for cancer therapeutics such as PARPi in the coming years.
- Additionally, the PARPi market is expected to experience growth as a result of the rising demand for PARPi due to its effectiveness. However, there are several obstacles that impede market progress, including the high expenses associated with clinical trials, strict regulatory standards, and frequent product recalls.
- For ovarian cancer, PARP inhibitors have revolutionized the care for patients with BRCA mutations and homologous recombination–deficient (HRD) mutations.
- There are promising opportunities for research in the field of PARP inhibitors. One such opportunity is to investigate biomarkers that can effectively identify patients who would derive the greatest benefits from PARP inhibitor treatment.
- Impact Therapeutics, Merck, Jiangsu Hengrui Pharmaceuticals, AstraZeneca, AtlasMedx, and several other companies are currently engaged in the development and production of selective PARP 1 inhibitors, which have the potential to significantly impact and enhance the PARPi market.
DelveInsight’s “Poly (ADP-ribose) Polymerase Inhibitors (PARPi) – Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the PARP inhibitors, historical and Competitive Landscape as well as the PARP inhibitors market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The PARP inhibitors market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM PARP inhibitors market size from 2020 to 2034. The report also covers current PARP inhibitor treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain), and the UK
- Japan
Study Period: 2020–2034
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
PARP Inhibitors Epidemiology |
Segmented by:
|
|
PARP Inhibitors Key Companies |
|
|
PARP Inhibitors Key Therapies |
|
|
PARP Inhibitors Market |
Segmented by:
|
|
PARP Inhibitors Market Analysis |
|
PARP Inhibitors Understanding
PARP Inhibitors Overview
Poly (ADP-ribose) polymerase (PARP) inhibitors are targeted therapies that block PARP enzymes, preventing cancer cells from repairing themselves and leading to cell death. They show promise, especially when combined with immune checkpoint inhibitors (ICIs), offering hope for many cancer patients. While generally specific and with manageable side effects, understanding the roles of PARP1/2 throughout the cell cycle is crucial for developing new therapies and anticipating potential side effects.
The treatment landscape for cancers with PARP inhibitors is rapidly evolving. Companies like Merck, AstraZeneca, and Waverley Pharma are focusing on PARP-1 selective inhibitors, currently in lead optimization, to meet unmet needs for various tumors. PARP inhibitors are used for certain stages and types of ovarian, pancreatic, and prostate cancers, particularly those with HR-DDR mutations.
In ovarian cancer, PARP inhibitors are combined with chemotherapy for advanced cases and recommended for recurrent epithelial ovarian cancer with specific genetic markers. In prostate cancer, PARP inhibitors are most effective in cases with BRCA1/2 mutations but only benefit a quarter of patients. Research continues into their use in pancreatic cancer. Notably, a 2018 trial showed olaparib's benefits for women with BRCA-mutated ovarian cancer.
|
Target of PARP inhibitors in the treatment of Pancreatic cancer | |
|
PARP inhibitor |
PARP target |
|
Olaparib |
PARP 1, 2 |
|
Rucaparib |
PARP 1, 2, 3 |
|
Talazoparib |
PARP 1, 2 |
|
Veliparib |
PARP 1, 2 |
|
Niraparib |
PARP 1, 2 |
|
Pamiparib (BGB 290) |
PARP 1, 2 |
Further details related to country-based variations are provided in the report
PARP Inhibitors Epidemiology
The PARP inhibitor epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Target pool (Incident Cases by Indication, Eligible and Treatable Cases by Indication) in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
|
Sr. No. |
Indications |
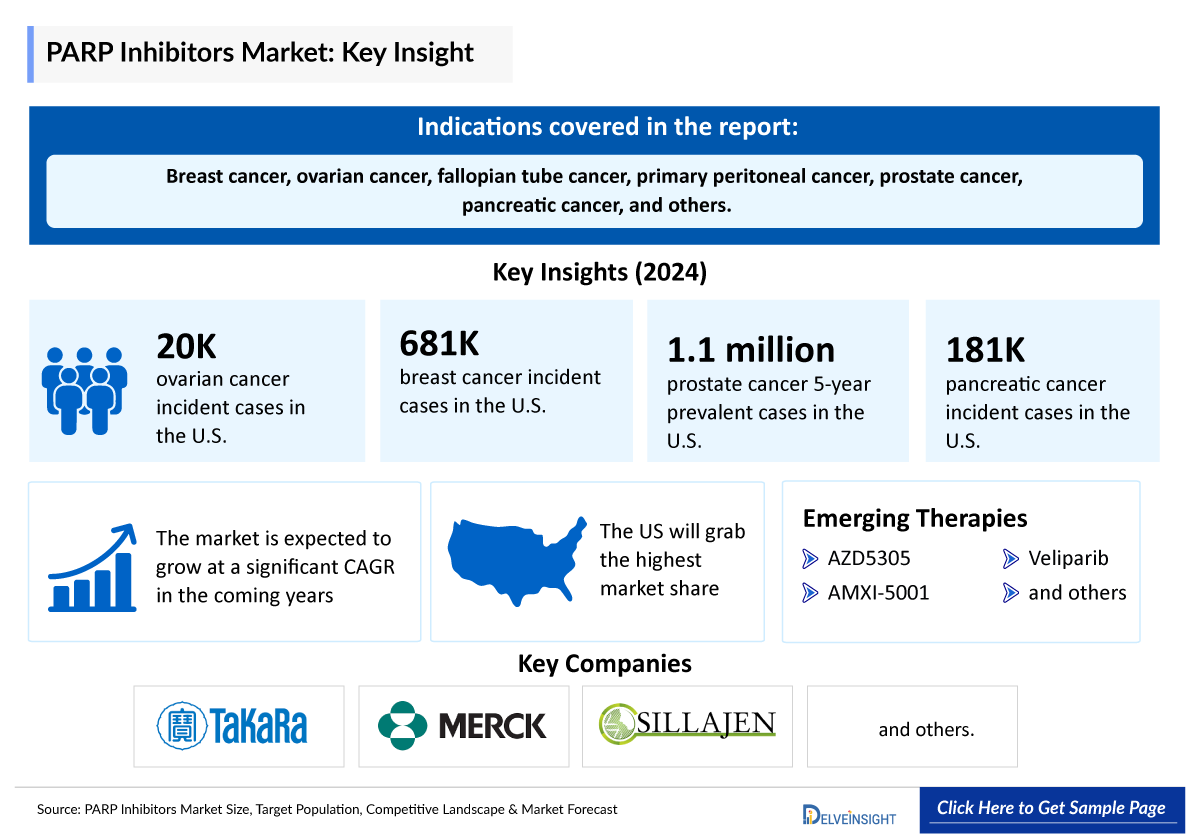
Estimated Cases in 2024 in the US |
|
1 |
Ovarian Cancer (including Fallopian Tube and Primary Peritoneal Cancers) |
~ 19,680 (Incident) |
|
2 |
Breast Cancer |
~681,500 (Incident) |
|
3 |
Prostate Cancer |
~ 1,123,900 (5 year prevalent) |
|
4 |
Pancreatic Cancer |
~181,000 (Incident) |
|
5 |
Small Cell Lung Cancer (SCLC) |
~94,700 (Incident) |
|
6 |
Gastric and Esophageal Cancers |
~299,700 (Incident) |
The total incident cases of breast cancer in the US comprised approximately 297,000 cases in 2023.
In 2023, in the United States, the total cases of metastatic castration-sensitive prostate cancer and metastatic castration-resistant prostate cancer were around 54,200 and 66,200, respectively.
Most cases of prostate cancer occur in people aged 54 years and older.
PARP Inhibitors Drug Chapters
The drug chapter segment of the PARP inhibitors reports encloses a detailed analysis of PARP inhibitors marketed drugs and late-stage (Phase III and Phase I/II) pipeline drugs. It also helps understand the PARP inhibitors' clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed Drugs
LYNPARZA (olaparib): AstraZeneca
LYNPARZA (olaparib) is the first and best-in-class oral poly ADP-PARP inhibitor and the first targeted treatment to block DDR in tumors harboring a deficiency in homologous recombination repair (HRR), such as mutations in BRCA1 and/or BRCA2. AstraZeneca has a global strategic oncology collaboration with Merck to co-develop and co-commercialize LYNPARZA.
LYNPARZA is a prescription medicine that is approved in many countries across multiple tumor types including maintenance treatment of platinum-sensitive relapsed ovarian cancer, for gBRCAm, HER2-negative high-risk early metastatic breast cancer, in combination with abiraterone for the treatment of metastatic castration-resistant prostate cancer and gBRCAm metastatic pancreatic cancer. LYNPARZA is solidifying its lead as the top-selling PARP inhibitor with a first-of-its-kind FDA approval.
ZEJULA (niraparib): GlaxoSmithKline
ZEJULA (niraparib) is an oral, potent, highly selective PARP1 and PARP2 inhibitor. PARP is a protein that plays a fundamental role in detecting and repairing DNA damage in cells, including damage induced by chemotherapy. Since Niraparib is a PARP inhibitor, it works by inhibiting the repair of damaged DNA and inducing cell death. Cells that are defective in the homologous recombination (HR) pathway are known as HRD cells and rely heavily on other repair proteins such as PARP to survive. ZEJULA continues to be an important maintenance treatment option for appropriate patients in the second-line or later setting and for patients who are in complete or partial response to first-line platinum-based chemotherapy.
|
List of Marketed Drugs | |||||
|
Product |
Company |
Ovarian Cancer |
Breast Cancer |
Pancreatic cancer |
Prostate Cancer |
|
LYNPARZA (olaparib) |
AstraZeneca |
● Maintenance of patients in response to 1st-line Patient chemo, BRCA+ ve ● Maintenance of patients in response to 1st-line Patient chemo, AVASTIN combo, HRD +ve ● Maintenance of patients in response to Patient chemotherapy |
● Adjuvant, post (neo) adjuvant chemo, HER2-ve, gBRCA +ve ● Post (neo) adjuvant chemo, HER2-ve, gBRCA +ve |
Maintenance of patients in response to 1st-line patients chemo, gBRCA +ve |
2nd-line, HRR +ve |
|
ZEJULA (niraparib) |
GlaxoSmithKline/Janssen Pharmaceutical |
● Maintenance of patients in response to 1st-line Patient chemo. ● Maintenance of patients in response to Patient chemotherapy. |
NA |
NA |
Treatment of adults with mCRPC and BRCA1/2 mutations (germline and/or somatic) in whom chemotherapy is not clinically indicated |
|
mCRPC: Metastatic Castration-Resistant Prostate Cancer gBRCAmut: germline BRCA | |||||
Note: Detailed current therapies assessment will be provided in the full report of PARP inhibitors
Emerging Drugs
AZD5305: AstraZeneca
AZD5305, a next-generation PARP1-selective inhibitor, in patients with tumors harboring specific homologous recombination repair gene mutations. AZD5305 is designed to selectively target PARP1, killing cancer cells by targeting tumor cell DNA damage repair mechanisms. This approach could allow PARP inhibitors to expand into new settings and offer new opportunities for combinations with DNA damage pathway activating agents such as ADCs. The next-generation PARP1 selective inhibitor, AZD5305, is progressing towards potential registrational trials for prostate cancer in combination with new hormonal agents, with data showing good tolerability at higher doses. Currently, the drug is being evaluated in clinical trials in patients with advanced solid tumors (Ovarian, breast, prostate, pancreatic cancer, and others).
|
Saruparib (AZD5305, PARP1 inhibitor) Data Anticipation | |||
|
Trial |
Population |
Phase |
Status |
|
NCT06120491 (EvoPAR-Prostate01)
|
HRRm and non-HRRm mCSPC |
Phase III |
Data anticipated: >2025 |
|
NCT04644068 (PETRA) |
Advanced solid tumors |
Phase I/IIa |
Data anticipated: >2025 |
|
NCT05367440 (PETRANHA) |
Metastatic prostate cancer |
Phase I/IIa
|
Data anticipated: >2025 |
Veliparib: AbbVie
Veliparib (ABT-888) is a potential anti-cancer drug acting as a PARP inhibitor. It kills cancer cells by blocking a protein called PARP, thereby preventing the repair of DNA or genetic damage in cancer cells and possibly making them more susceptible to anticancer treatments. It inhibits both PARP1 and PARP2 and thereby induces synthetic lethality. It is still being evaluated for the treatment of solid tumors.
Note: Detailed emerging therapies assessment will be provided in the final report.
|
List of Emerging Drugs | ||||||
|
Drug Name |
Company |
Condition |
MoA |
Phase |
NCT Number | |
|
AZD5305 |
AstraZeneca |
Advanced solid tumors (Ovarian, breast, prostate, pancreatic cancer, and others) |
PARP1 inhibitor
|
I/IIa |
NCT04644068 (PETRA) | |
|
Metastatic Prostate Cancer |
I/IIa |
NCT05367440 (PETRANHA) | ||||
|
AMXI-5001 |
AtlasMedx |
Advanced Malignancies (Ovarian, breast, prostate, pancreatic cancer) |
PARP1/2 inhibitor |
I/II |
NCT04503265 | |
|
Veliparib |
AbbVie |
Metastatic Breast Cancer |
PARP1 and PARP2 inhibitor |
II |
NCT01009788 | |
Note: The emerging drug list is indicative, the full list will be given in the final report.
PARP Inhibitors Market Outlook
The market for PARP inhibitors are expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness of PARP inhibitors, and the increasing number of PARP inhibitors that are being approved by the FDA.
To date, approved PARP inhibitors include LYNPARZA (olaparib), TALZENNA (talazoparib), ZEJULA (niraparib), and RUBRACA (rucaparib). Currently, LYNPARZA is dominating the PARP inhibitors market.
PARPi monotherapy has been a milestone in the management of many BRCA1/2-mutated cancers, offering patients and prescribers the hope of effective therapy options. Alternate therapeutic pathways for PARPi and potential mechanisms of sensitivity and resistance remain major areas of current clinical research. Biomarkers are critical in identifying optimal patient populations because although the effect is beneficial in tumors with BRCA1/2 mutations or HRD, HR-proficient markers have yet to be determined.
Before restricting the use of ZEJULA in second-line maintenance ovarian cancer indication in the US to only cover patients with germline BRCA mutations, GSK already pulled ZEJULA’s approval in late-line ovarian cancer. GSK's decision could create a cascade of impacts, potentially reverberating across the landscape of PARP inhibitors. This move might have implications for other drugs in the same class, such as AstraZeneca and Merck's LYNPARZA, as well as Clovis Oncology's RUBRACA.
RUBRACA's primary source of revenue at the moment is its utilization as a second-line maintenance treatment for ovarian cancer. However, if there were limitations imposed on its label specifically for this indication, it would significantly increase the risk of Clovis, the company behind RUBRACA, facing potential bankruptcy.
The promising results from the clinical trial have positioned TALZENNA as a potential competitor to two other well-known PARP inhibitors: LYNPARZA by AstraZeneca and Merck, and ZEJULA by GSK. However, the success of TALZENNA in treating mCRPC may not solely rely on its own merits but could be further enhanced by its therapeutic partner, Xtandi. Xtandi is currently a leading product in the field of prostate cancer treatment.
Several key players, including AstraZeneca, Allarity Therapeutics, AtlasMedx, BeiGene, and others, are involved in developing drugs for PARP inhibitors for various indications such as Ovarian cancer, breast cancer, prostate cancer, pancreatic cancer, and others.
Overall, this is an exciting class of agents with great potential for development. Maturation of current studies over the next few years will lead to a better understanding of PARP inhibitors and define their role in the therapy of cancer.
PARP inhibitors Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging PARP inhibitors expected to be launched in the market during 2020–2034. LYNPARZA continues to be the most widely prescribed medication in the PARP inhibitor family for four tumor types. It is being used for conditions like breast, ovarian, and prostate cancer. Increasing rates of HRD testing and utilization in first-line HRD-positive ovarian cancer in Europe. Additionally, LYNPARZA’s uptake has increased in advanced HER2-negative breast cancer and BRCAm mCRPC.
PARP Inhibitors Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics. The presence of numerous drugs under different stages is expected to generate immense opportunity for PARPi market growth over the forecasted period.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for PARP inhibitors emerging therapies.
The increasing strategic collaborations among major market players to enhance the growth of their pipeline products are anticipated to drive market expansion. For example, in January 2019, GSK completed the acquisition of TESARO, enabling the expansion of its ovarian cancer portfolio. This acquisition grants GSK marketing rights for ZEJULA, a treatment for ovarian cancer.
KOL Views
To keep up with current and future market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on PARP inhibitors evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers such as MD Anderson Cancer Center, Texas from UT Southwestern Medical Center in Dallas, Cancer Research UK Barts Centre in London, MD Anderson Cancer Center, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or PARP inhibitors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
KOL Views |
|
“PARP inhibitors have proven quite effective not just in patients with recurrent disease but also in those who are newly diagnosed, and they have made maintenance therapy a reality — something that has been a long-sought goal in ovarian cancer." |
|
“BRCA1- and BRCA2-mutated tumors are sensitive to PARP inhibitors and platinum agents because they are deficient in homologous recombination repair of DNA damage. The effectiveness of PARP inhibitors demonstrates proof of principle that targeting the DNA repair pathway is a productive treatment strategy” |
|
“As a global standard of care, Xtandi has shown efficacy in three types of prostate cancer, and the addition of TALZENNA demonstrated significant improvements in delaying or preventing radiographic progression-free survival or death in patients with this type of advanced prostate cancer. With today’s FDA approval of TALZENNA plus Xtandi, we are proud to be able to offer this potentially practice-changing treatment to patients and add to their options in managing this aggressive disease”. |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Market Access and Reimbursement
Reimbursement for PARP inhibition has been universal in the United States and even in Europe. However, there is a specific requirement for reimbursement in the case of the bevacizumab-olaparib combination, where a companion diagnostic is necessary. Generally, reimbursement is limited to patients who exhibit molecular HRD (homologous recombination deficiency). As oral cancer therapies, PARP inhibitors are typically covered under pharmaceutical drug benefits, often falling under specialty drug tiers. In the case of Medicare Part D beneficiaries, there is a risk of significant out-of-pocket spending on drugs as there is no absolute limit on such expenses. Research has shown that higher patient out-of-pocket costs for oral cancer therapies can lead to increased rates of prescription abandonment, delayed treatment initiation, and non-adherence. To alleviate some of the financial burden associated with these expensive oral medications, companies often provide support through copay assistance programs and foundations. However, there remains a considerable financial burden for patients.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
2024 Updates on PARP Inhibitors
Results presented at ASCO from a Phase I study investigating perioperative fuzuloparib in combination with mFOLFIRINOX for resectable pancreatic adenocarcinoma demonstrated that this regimen was generally well-tolerated and exhibited an acceptable safety profile in patients. Additionally, neoadjuvant fuzuloparib combined with mFOLFIRINOX prior to surgery achieved a remarkable R0 resection rate of 100%.
Findings presented during the 2024 ESMO Breast Cancer Congress showed that a combination of LYNPARZA, IMFINZI and FASLODEX demonstrated clinical activity and an acceptable toxicity profile when administered as a second- or third-line treatment to patients with primarily pretreated, endocrine-resistant ER-positive, HER2-negative metastatic breast cancer who expressed molecular abnormalities associated with PARP inhibitor sensitivity.
The first-in-class PARP1 inhibitor saruparib generated a favorable safety profile alongside durable responses, tumor reductions, and encouraging progression-free survival at 60 mg in heavily pretreated patients with breast cancer expressing homologous recombination repair mutations, according to findings from the Phase I/IIa PETRA study (NCT04644068) presented during the 2024 AACR Annual Meeting.
In January 2024, Pfizer announced that the European Commission (EC) approved TALZENNA (talazoparib) in combination with XTANDI (enzalutamide) for the treatment of adult patients with metastatic castration-resistant prostate cancer (mCRPC) in whom chemotherapy is not clinically indicated. With this approval, TALZENNA is now the first and only PARP inhibitor licensed in the European Union for use with XTANDI for patients with mCRPC, with or without gene mutations.
Abstract list is not exhaustive, will be provided in the final report
Scope of the Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of PARP inhibitors, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the competitive landscape, and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the PARP inhibitors market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM PARP inhibitors market.
PARP inhibitors report insights
- PARP Targeted Patient Pool
- PARP Inhibitors Pipeline Analysis
- PARP Inhibitors Market Size and Trends
- Existing and future Market Opportunity
PARP inhibitors report key strengths
- Eleven years Forecast
- The 7MM Coverage
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
PARP inhibitors report assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT)
Key Questions
- What was the PARP inhibitor total market size, the market size by therapies, market share (%) distribution in 2023, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative market for PARP inhibitors?
- Which indication accounts for maximum PARP inhibitors sales?
- What are the pricing variations among different geographies for approved therapies?
- How has the reimbursement landscape for PARP inhibitors evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to PARP inhibitors?
- What are the key factors hampering the growth of the PARP inhibitors market?
- What are the recent novel therapies, targets, and technologies developed to overcome the limitations of existing therapies?
- What key designations have been granted for the emerging therapies for PARP inhibitors?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the PARP inhibitors market.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.