Peanut Allergy Pipeline Insight
DelveInsight’s, “Peanut Allergy - Pipeline Insight, 2025,” report provides comprehensive insights about 12+ companies and 15+ pipeline drugs in Peanut Allergy pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Geography Covered
- Global coverage
Peanut Allergy Understanding
Peanut Allergy: Overview
Peanut allergy is one of the most common and severe food allergies, particularly in children, and poses a significant public health concern due to its potential to trigger life-threatening anaphylactic reactions. It occurs when the immune system mistakenly identifies peanut proteins as harmful, leading to an exaggerated immune response upon exposure. Even trace amounts of peanuts can provoke symptoms ranging from mild hives and swelling to severe breathing difficulties and cardiovascular complications. The condition often develops early in life and tends to persist into adulthood, necessitating strict dietary avoidance and emergency preparedness, including the use of epinephrine auto-injectors.
Diagnosis of a peanut allergy relies on patient history and physical exam. The type/amount of food ingested, onset/duration of symptoms, and relieving factors are key in making an accurate diagnosis. A history of eczema is important as it is a significant risk factor for peanut sensitization. Common symptoms of peanut allergy include skin reactions, such as urticaria, erythema, or edema. More severe symptoms include tingling of the mouth and throat, edema of the lips, and dyspnea. These symptoms will often frequently progress to anaphylaxis.
Skin testing is the preferred testing method in those patients with a history compatible with food allergies. A skin-prick test is performed by applying a drop of a peanut extract to the skin, commonly on the arm or back. A wheal/flare response is measured approximately 20 minutes after pricking the skin where the drop of peanut extract was placed. Positive testing indicates sensitization to peanuts and can predict the probability of a future reaction. Measurement of peanut-specific IgE antibodies in serum is another useful method to estimate sensitization and the probability of future reaction. It is important to note that this type of testing does not predict the severity of the reaction and is limited by poor sensitivity; results are reassuring when negative. Ultimately a clinician-supervised oral food challenge is the best and most reliable method for diagnosing peanut allergy. Serum-specific IgE and skin-prick testing can aid in determining which patients should have an oral food challenge. An oral food challenge can also assist in confirming reactivity in those patients without a clear history or never having ingested peanuts despite sensitization. Efforts to address peanut allergies include ongoing research into immunotherapy treatments, such as oral immunotherapy, which aims to desensitize individuals to peanuts by gradually exposing them to controlled doses.
"Peanut Allergy - Pipeline Insight, 2025" report by DelveInsight outlays comprehensive insights of present scenario and growth prospects across the indication. A detailed picture of the Peanut Allergy pipeline landscape is provided which includes the disease overview and Peanut Allergy treatment guidelines. The assessment part of the report embraces, in depth Peanut Allergy commercial assessment and clinical assessment of the pipeline products under development. In the report, detailed description of the drug is given which includes mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, Peanut Allergy collaborations, licensing, mergers and acquisition, funding, designations and other product related details.
Report Highlights
- The companies and academics are working to assess challenges and seek opportunities that could influence Peanut Allergy R&D. The therapies under development are focused on novel approaches to treat/improve Peanut Allergy.
Peanut Allergy Emerging Drugs Chapters
This segment of the Peanut Allergy report encloses its detailed analysis of various drugs in different stages of clinical development, including phase III, II, I, preclinical and Discovery. It also helps to understand clinical trial details, expressive pharmacological action, agreements and collaborations, and the latest news and press releases.
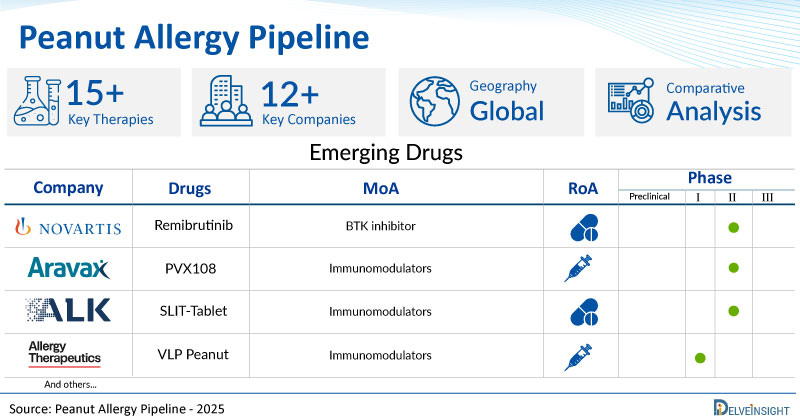
Peanut Allergy Emerging Drugs
- Remibrutinib: Novartis
Remibrutinib (LOU064), an oral, novel, covalent BTK inhibitor, has shown high selectivity and potency for BTK, which is implicated in CSU. It is an oral BTK inhibitor that blocks the BTK cascade and prevents the release of histamine that causes itchy hives (wheals) and swelling. The high selectivity and tolerability of remibrutinib are likely attributable to its ability to bind to an inactive conformation of BTK. Currently, the drug is being investigated in Phase II Stage of clinical investigation for Peanut Allergy.
- VLP Peanut: Allergy Therapeutics
VLP Peanut (Polyvac peanut) vaccine is under development for the prevention of peanut allergy. The vaccine candidate is administered by subcutaneous and intravenous route. It consists of a recombinant peanut allergen coupled with a virus-like particle (VLP) adjuvant. It is developed based on virus-like particles (VLP) technology. VLP Peanut consists of two proteins: a capsid subunit derived from Cucumber mosaic virus engineered with a universal T-cell epitope (CuMVTT ) and a CuMVTT subunit fused with peanut allergen Ara h 2 (CuMVTT -Ara h 2), forming mosaic VLPs. Currently, the drug is being investigated in Phase I Stage of clinical investigation for Peanut Allergy.
- INT301: Intrommune Therapeutics
INT301 is the initial product in development by Intrommune specifically designed to help those who suffer from peanut allergy. The product is intended to significantly raise a patient’s immune threshold through daily use of OMIT toothpaste beyond what has triggered a potentially dangerous allergic reaction via accidental exposure. This additional protection helps relieve the persistent anxiety of peanut allergic individuals toward accidental exposure. Currently, the drug is being investigated in Phase I Stage of clinical investigation for Peanut Allergy.
Further product details are provided in the report……..
Peanut Allergy: Therapeutic Assessment
This segment of the report provides insights about the different Peanut Allergy drugs segregated based on following parameters that define the scope of the report, such as:
- Major Players in Peanut Allergy
There are approx. 12+ key companies which are developing the therapies for Peanut Allergy. The companies which have their Peanut Allergy drug candidates in the most advanced stage, i.e. phase II include, Novartis.
Phases
DelveInsight’s report covers around 15+ products under different phases of clinical development like
- Late stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
Route of Administration
Peanut Allergy pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
- Oral
- Parenteral
- intravenous
- Subcutaneous
- Topical.
Molecule Type
Products have been categorized under various Molecule types such as
- Monoclonal Antibody
- Peptides
- Polymer
- Small molecule
- Gene therapy
Product Type
Drugs have been categorized under various product types like Mono, Combination and Mono/Combination.
Peanut Allergy: Pipeline Development Activities
The report provides insights into different therapeutic candidates in phase III, II, I, preclinical and discovery stage. It also analyses Peanut Allergy therapeutic drugs key players involved in developing key drugs.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing along with a thorough therapeutic assessment of emerging Peanut Allergy drugs.
Peanut Allergy Report Insights
- Peanut Allergy Pipeline Analysis
- Therapeutic Assessment
- Unmet Needs
- Impact of Drugs
Peanut Allergy Report Assessment
- Pipeline Product Profiles
- Therapeutic Assessment
- Pipeline Assessment
- Inactive drugs assessment
- Unmet Needs
Key Questions
Current Treatment Scenario and Emerging Therapies:
- How many companies are developing Peanut Allergy drugs?
- How many Peanut Allergy drugs are developed by each company?
- How many emerging drugs are in mid-stage, and late-stage of development for the treatment of Peanut Allergy?
- What are the key collaborations (Industry–Industry, Industry–Academia), Mergers and acquisitions, licensing activities related to the Peanut Allergy therapeutics?
- What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Peanut Allergy and their status?
- What are the key designations that have been granted to the emerging drugs?