Peripheral Arterial Disease (PAD)/ Pulmonary Vascular Disease (PVD) Market
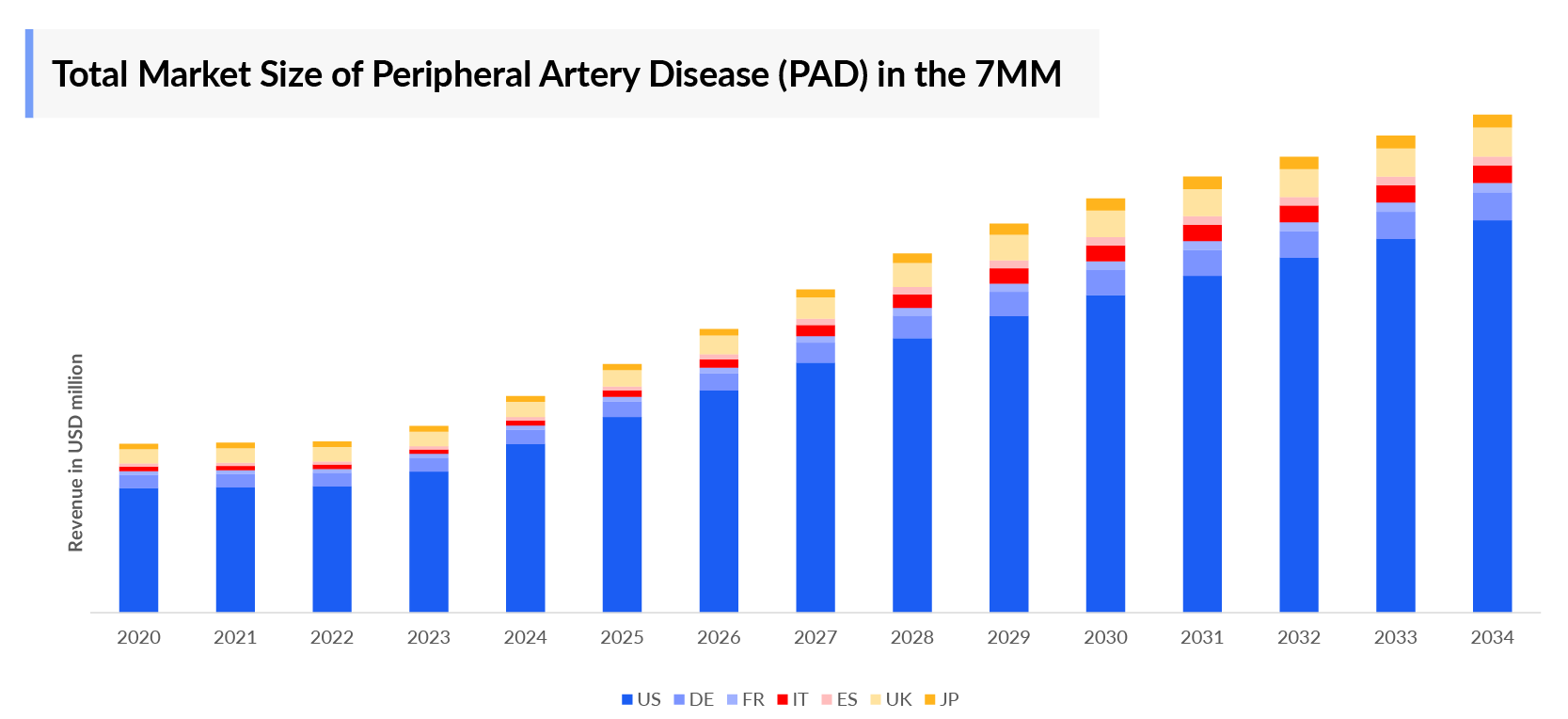
- In 2023, the market size of Peripheral Artery Disease (PAD) was highest in the US among the 7MM, accounting for approximately USD 2,665 million which is further expected to increase by 2034.
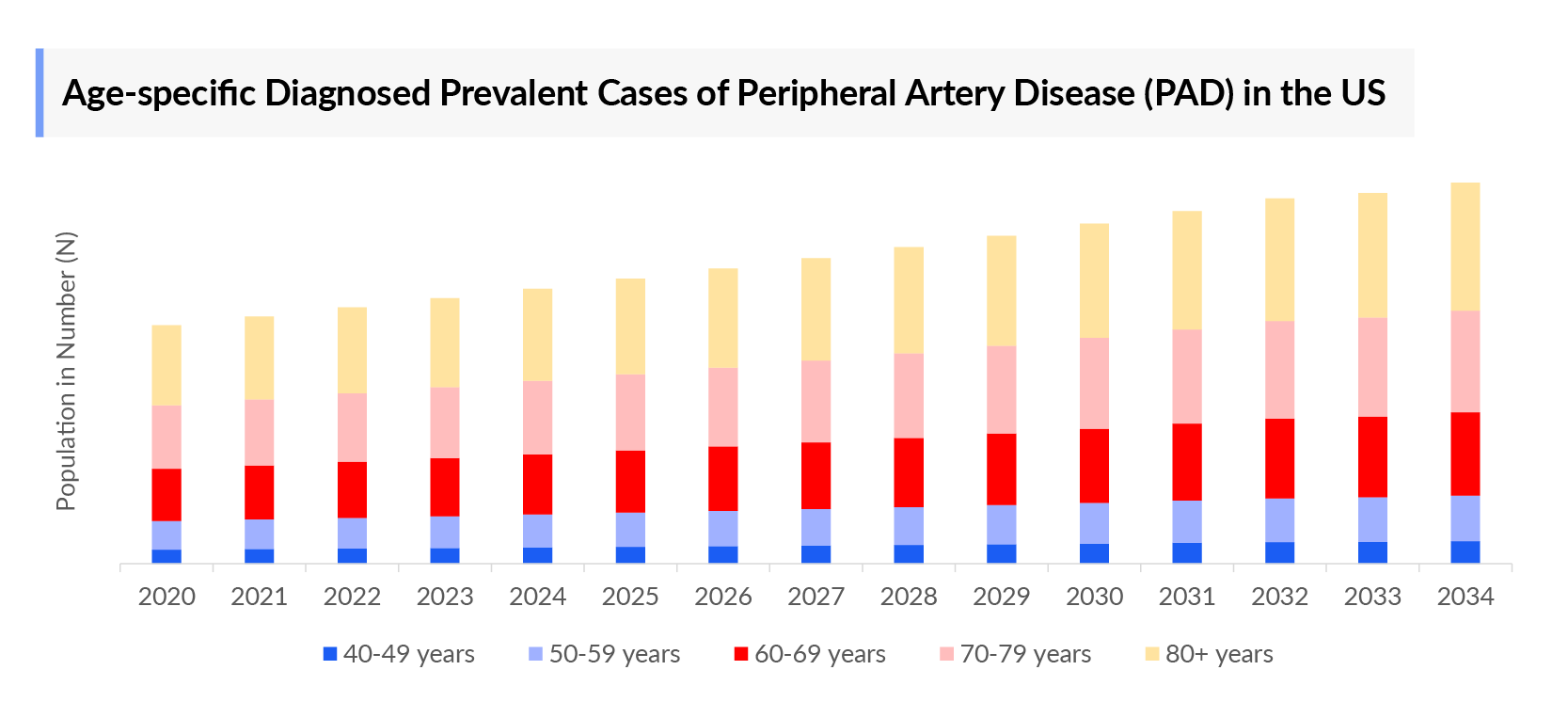
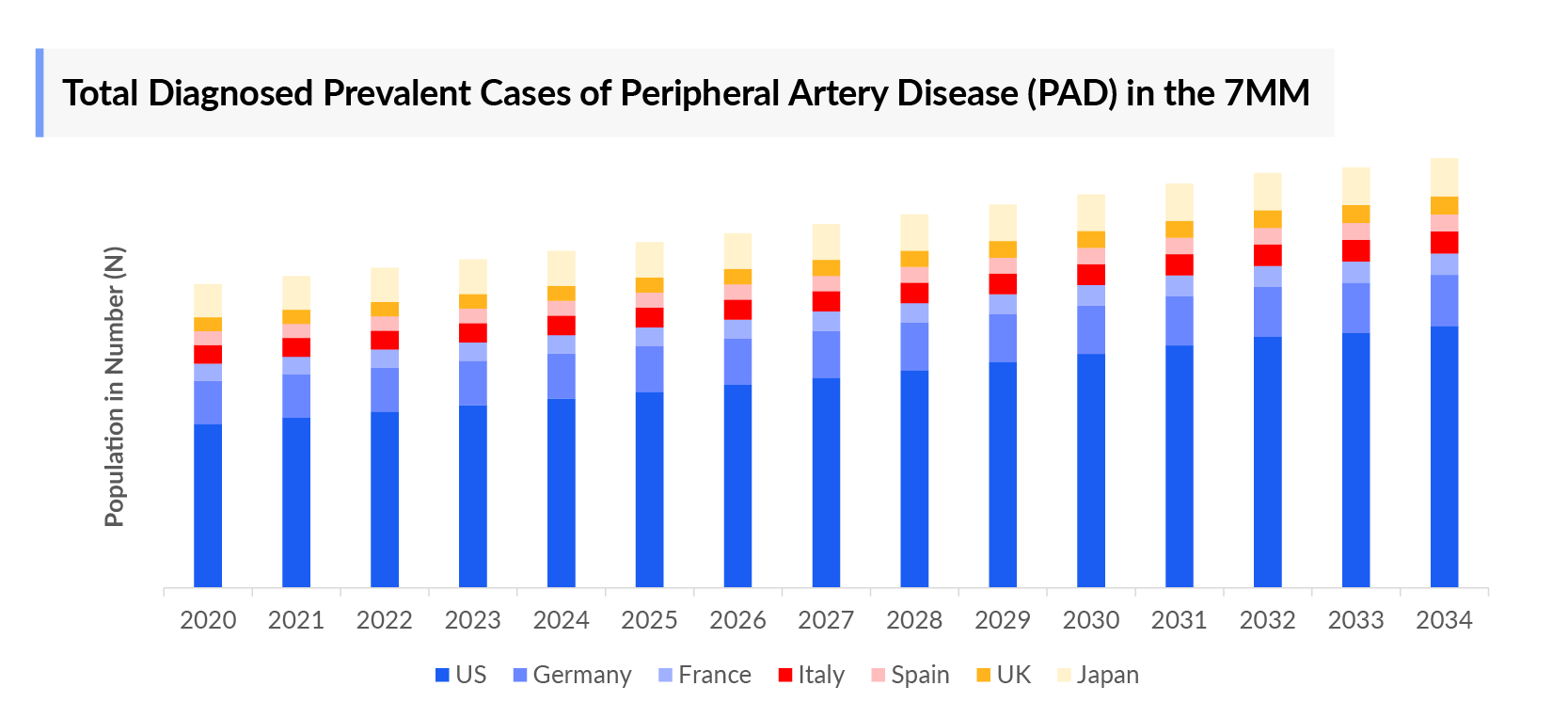
- In 2023, the diagnosed prevalence of Peripheral Artery Disease (PAD) was highest in the US among the 7MM, accounting for nearly 10 million cases which is further expected to increase by 2034.
- In the United States, in 2023, ~4.6 million males and ~5.2 million females were affected with Peripheral Artery Disease (PAD).
- The market size was of nearly USD 4,055 million in the 7MM in 2023. The market size is expected to increase with the projected launch of emerging therapies during the forecast period (2024–2034).
- The market size of Peripheral Artery Disease (PAD) in Japan was around USD 387 million in 2023, which is expected to rise at a significant CAGR by 2034.
- Several major pharma and biotech companies such as NovoNordisk, Takeda, Mercator MedSystems, Inc., Beijing Northland Biotech. Co., Ltd., Ixaka Ltd, Humacyte, Inc., CardioVascular BioTherapeutics, Proteon Therapeutics, ReNeuron Limited, Alucent Biomedical, Athersys, ARCA biopharma, Ambulero, and Venturis Therapeutics, among others, are actively working in the Peripheral Arterial Disease Market.
DelveInsight’s “Peripheral Artery Disease Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of the Peripheral Artery Disease (PAD) , historical and forecasted epidemiology as well as the Peripheral Artery Disease (PAD) market trends in the United States, EU4 and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The Peripheral Artery Disease (PAD) market report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Peripheral Artery Disease (PAD) market size from 2020 to 2034. The Report also covers current Peripheral Artery Disease (PAD) treatment practices, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
Peripheral Artery Disease Treatment Market
Peripheral Artery Disease (PAD) Overview
Peripheral artery disease is a common manifestation of arterial stenosis of the lower and/or upper extremities that reduces arterial flow. It is well known that PAD is associated with elevated morbidity and mortality with cardiovascular (CV) disease. In PAD, the legs or arms—usually the legs—do not receive enough blood flow to keep up with the demand, which may cause leg pain when walking (claudication) and other symptoms.
Many people with PAD have no symptoms. The most common symptom of lower-extremity PAD is painful muscle cramping in the hips, thighs, or calves when walking, climbing stairs, or exercising. However, those who develop a painful ache in their legs when they walk usually disappear after a few minutes of rest. The medical term for this is “intermittent claudication”. The symptoms of PAD often develop slowly over time. If your symptoms develop quickly or get suddenly worse, it could be a sign of a serious problem requiring immediate treatment.
Peripheral Artery Disease (PAD) Diagnosis
Peripheral Artery Disease (PAD) is typically diagnosed through a combination of patient history, physical examination, and diagnostic testing. The initial assessment involves a thorough review of symptoms, such as leg pain while walking (claudication), numbness, or non-healing wounds. Physical examination may reveal weak or absent pulses in the legs, cool skin, or poor wound healing.
Ankle-Brachial Index (ABI) is a common non-invasive test used to diagnose PAD. It compares the blood pressure in the ankle with the blood pressure in the arm. An ABI value of less than 0.9 indicates PAD. Doppler ultrasound can further evaluate blood flow and identify blockages in the arteries.
For more detailed imaging, tests such as magnetic resonance angiography (MRA) or computed tomography angiography (CTA) can be used. These tests provide high-resolution images of the blood vessels and help in pinpointing the exact location and severity of arterial blockages.
In some cases, invasive angiography might be necessary. This involves injecting a contrast dye into the arteries and taking X-rays to visualize blood flow and identify blockages. Early diagnosis and management of PAD are crucial to prevent complications such as heart attack, stroke, and limb amputation.
Further details related to diagnosis are provided in the report...
Peripheral Artery Disease (PAD) Treatment
There is no cure for PAD, but lifestyle changes and medicine can help reduce the symptoms. Medications called statins are commonly prescribed. If PAD is so far progressed and does not respond to noninvasive therapies such as lifestyle changes, medication, or both, surgery may be necessary.
Medical therapy aims to reduce the risk of future cardiovascular morbidity and mortality in patients with high ischemic risk. Secondary prevention includes using antiplatelet agents and angiotensin-converting enzyme (ACE) inhibitors and managing other risk factors such as tobacco use, diabetes, low-density lipoprotein levels, and hypertension. Three main treatment options for improving functional status in patients with intermittent claudication are exercise training, medical therapy, and revascularization.
Many cell therapies are in the development pipeline to target the population who are not suitable for revascularization or showed poor results after revascularization which might change the dynamics of current treatment market in 7MM countries.
Further details related to treatment are provided in the report...
Peripheral Artery Disease (PAD) Epidemiology
As the market is derived using a patient-based model, the Peripheral Artery Disease (PAD) epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by, Total Diagnosed Prevalent Cases of PAD, Gender-specific Diagnosed Prevalent Cases of PAD, Age-specific Diagnosed Prevalent Cases of PAD, and Severity-specific Diagnosed Prevalent Cases of PAD in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- In the assessment done by DelveInsight, the estimated total diagnosed prevalent cases of PAD in the 7MM were nearly 18 million in 2023.
- Among the European countries, Germany had the highest diagnosed prevalent cases of PAD with ~2.4 million cases. On the other hand, Spain had the lowest diagnosed prevalent population (0.8 million cases).
- Japan had nearly 2 million total diagnosed prevalent cases of Peripheral artery disease PAD in 2023, accounting for approximately 10% in 7MM.
- Based on Rutherford’s criteria for severity, the highest number of PAD cases in 2023 were observed in Stage 0 i.e., 56% in the US, while Stage 6 patients were lowest (2%).
- The DelveInsight analysis indicates that among the EU4 and the UK, the PAD prevalence in females (51%) is slightly high compared to males (49%).
Peripheral Artery Disease (PAD) Recent Developments
- On November 4, 2024, U.S.-based R3 Vascular received investigational device exemption (IDE) approval from the FDA to initiate the pivotal ELITE-BTK trial, aimed at evaluating its bioabsorbable scaffold treatment for peripheral arterial disease (PAD). This trial represents a significant step forward for R3 Vascular in developing innovative therapies for PAD patients.
Peripheral Artery Disease (PAD) Drug Chapters
The drug chapter segment of the Peripheral Artery Disease (PAD) report encloses a detailed analysis of Peripheral Artery Disease (PAD) off-label drugs and late-stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Peripheral Artery Disease (PAD) clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Peripheral Artery Disease Marketed Drugs
XARELTO (rivaroxaban): Bayer/ Johnson & Johnson
XARELTO is an oral anticoagulant for the treatment and prevention of blood clots. In combination with aspirin, it is indicated to reduce the risk of major thrombotic vascular events (myocardial infarction, ischemic stroke, acute limb ischemia, and major amputation of a vascular etiology) in adult patients with PAD, including patients who have recently undergone a lower extremity revascularization procedure due to symptomatic PAD. XARELTO can cause bleeding, which can be serious and may lead to death, as it is a blood thinner medicine (anticoagulant) that lowers blood clotting. In June 2022, the Ministry of Health, Labour and Welfare in Japan approved XARELTO (2.5 mg twice daily, used in combination with aspirin 81–100 mg once daily) to treat patients with PAD after revascularization.
ZONTIVITY (Vorapaxar): Xspire Pharma/ Aralez Pharmaceuticals/ Merck
ZONTIVITY is indicated for reducing thrombotic cardiovascular events in patients with a history of MI or with PAD. ZONTIVITY has been shown to reduce the rate of a combined endpoint of cardiovascular death, MI, stroke, and UCR. In May 2014, Merck received US FDA approval for ZONTIVITY to treat patients with PAD
Emerging Peripheral Artery Disease Drugs
Honedra (LSTA12): Lisata Therapeutics
Critical limb ischemia (CLI) is a severe obstruction of the arteries which markedly reduces blood flow to the extremities (hands, feet, and legs). CLI is the advanced form of peripheral arterial disease (PAD) caused by atherosclerosis, the hardening and narrowing of the arteries over time due to the buildup of fatty deposits called plaque. LSTA12 (also known as Honedra and CLBS12 in Japan) is an experimental regenerative medicine to prevent the serious adverse consequences of CLI and Buerger’s disease by improving blood flow in the affected limb. It is CD34+ cell therapy intended to administer by Intramuscular route, 20 injections in the target limb in a single treatment.
The CD34+ cell was discovered due to the deliberate search for a stem cell capable of stimulating blood vessels’ development and/or repair. All tissues in the human body maintain their function by replacing cells over time. By administering CD34+ cells, the company promotes the development and formation of new microvasculature, thereby increasing blood flow to the impacted area. No other native cell discovered to date has demonstrated this same capability. Currently the therapy is in Phase II of clinical development.
ACP-01: Hemostemix Inc..
ACP-01, Hemostemix’s lead clinical candidate, is an autologous cell therapy to treat critical limb ischemia (CLI) in patients facing amputation.
ACP-01 consists of cells derived from the patient’s blood and modified with Hemostemix’s technology that forms new blood vessels. These cells, known as angiogenic cell precursors, or ACPs, secrete growth factors and cytokines that support the formation of blood vessels through vasculogenesis and angiogenesis. Factors secreted by ACPs also facilitate the recruitment of additional progenitor cells to promote the healing of damaged tissues. When these cells are injected into the dying leg muscle of the same patient with critical limb ischemia, they support the regeneration of new small blood vessels and may prevent amputations.
Note: Detailed emerging therapies assessment will be provided in the final report of Peripheral artery disease...
Peripheral Artery Disease (PAD) Market Outlook
Peripheral Artery Disease (PAD) presents various limb-related complications, including intermittent claudication, ischemic rest pain, ulcers, gangrene, and functional impairment. Comprehensive medical treatment involves lowering cholesterol, antiplatelet therapy, anticoagulation, peripheral vasodilators, blood pressure control, exercise, and smoking cessation. Treatment options include lifestyle changes, medication, endovascular and surgical interventions, and emerging therapies like gene and cell therapy. Despite available treatments, PAD is often undertreated compared to coronary artery disease. Critical Limb Ischemia (CLI) treatment aims to relieve pain, restore blood flow, and preserve limb function. Strategies include endovascular and surgical revascularization, with therapeutic angiogenesis using gene and cell therapies showing promise. The goals for CLI management are pain relief, healing of lesions, prevention of amputations, and improvement of quality of life and survival. An interdisciplinary approach is essential, with monitoring and managing cardiovascular risk factors being crucial for all PAD patients.
- The market size of Peripheral Artery Disease (PAD) in EU4 and the UK was ~USD 1,004 million in 2023, which is further anticipated to increase during the forecast period.
- The United States accounted for the highest market size of PAD approximately 66% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the European countries, Germany had the highest market size with nearly USD 402 million each in 2023, while Spain had the lowest market size for Peripheral Artery Disease (PAD) with USD ~133 million in 2023.
- With the expected launch of upcoming therapies, such as Honedra (LSTA12) and ACP-01 among others, the total market size of Peripheral Artery Disease (PAD) is expected to show change in the upcoming years.
Peripheral Artery Disease (PAD) Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to launch in the market during 2020–2034. For example, ACP-01 in the US is expected to be launched by 2026 with a peak share of 2.6%. ACP-01 is anticipated to take 7 years to peak with a medium uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Peripheral Artery Disease (PAD) Pipeline Development Activities
The report provides insights into Peripheral Artery Disease clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Peripheral Artery Disease (PAD) emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Peripheral Artery Disease (PAD) evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from Fireman Vascular Center, Massachusetts General Hospital, Boston, US, School of Public Health, West Virginia University, Morgantown, US, Yale University School of Medicine, New Haven, CT, US; Gefässzentrum, Asklepios Westklinikum Hamburg, Germany; University of Palermo, Palermo, Italy; Institut Municipal d’Investigacio´ Me`dica (IMIM), Barcelona, Spain; Royal Liverpool University Hospital, Prescot Street, Liverpool, UK and Department of Surgery, the University of Tokyo, Tokyo, Japan; and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Peripheral Artery Disease (PAD) market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Peripheral Artery Disease Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Peripheral Artery Disease (PAD), explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Peripheral Artery Disease (PAD) market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Peripheral Artery Disease (PAD) market.
Peripheral Artery Disease (PAD) Report Insights
- Peripheral Artery Disease Patient Population
- Peripheral Artery Disease Therapeutic Approaches
- Peripheral Artery Disease (PAD) Pipeline Analysis
- Peripheral Artery Disease (PAD) Market Size and Trends
- Existing and Future Market Opportunity
Peripheral Artery Disease (PAD) Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Peripheral Artery Disease (PAD) Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Peripheral Artery Disease Drugs Uptake
- Key Peripheral Artery Disease Market Forecast Assumptions
Peripheral Artery Disease (PAD) Report Assessment
- Current Peripheral Artery Disease Treatment Practices
- Peripheral Artery Disease Unmet Needs
- Peripheral Artery Disease Pipeline Product Profiles
- Peripheral Artery Disease Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Peripheral Artery Disease Market Drivers
- Peripheral Artery Disease Market Barriers
Key Questions Answered In The Peripheral Artery Disease Market Report
Peripheral Artery Disease Market Insights
- What was the Peripheral Artery Disease (PAD) market share (%) distribution in 2020 and how it would look like in 2034?
- What would be the Peripheral Artery Disease (PAD) total market size as well as market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Peripheral Artery Disease (PAD) market size during the forecast period (2024–2034)?
- At what CAGR, the Peripheral Artery Disease (PAD) market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the Peripheral Artery Disease (PAD) market outlook across the 7MM during the forecast period (2024–2034)?
- What would be the Peripheral Artery Disease (PAD) market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Peripheral Artery Disease Epidemiology Insights
- What is the disease risk, burden, and unmet needs of Peripheral Artery Disease (PAD)?
- What is the historical Peripheral Artery Disease (PAD) patient population in the United States, EU5 (Germany, France, Italy, Spain, and the UK), and Japan?
- What would be the forecasted patient population of Peripheral Artery Disease (PAD) at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Peripheral Artery Disease (PAD)?
- Out of the above-mentioned countries, which country would have the highest prevalent population of Peripheral Artery Disease (PAD) during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current Peripheral Artery Disease Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Peripheral Artery Disease (PAD) along with the approved therapy?
- What are the current treatment guidelines for the treatment of Peripheral Artery Disease (PAD) in the US, Europe, And Japan?
- What are the Peripheral Artery Disease (PAD) marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, and efficacy, etc.?
- How many companies are developing therapies for the treatment of Peripheral Artery Disease (PAD)?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Peripheral Artery Disease (PAD)?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Peripheral Artery Disease (PAD) therapies?
- What are the recent therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Peripheral Artery Disease (PAD) and their status?
- What are the key designations that have been granted for the emerging therapies for Peripheral Artery Disease (PAD)?
- What are the 7MM historical and forecasted market of Peripheral Artery Disease (PAD)?
Reasons to Buy Peripheral Artery Disease Market Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Peripheral Artery Disease (PAD) Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.
Frequently Asked Questions
1. What is the forecast period covered in the report?
The Peripheral Artery Disease (PAD) Epidemiology and Market Insight report for the 7MM covers the forecast period from 2024 to 2034, providing a projection of market dynamics and trends during this timeframe.
2. Who are the key players in the Peripheral Artery Disease (PAD) market?
The Peripheral Artery Disease (PAD) market is quite robust. The major layers are Bayer/ Johnson & Johnson, Xspire Pharma/ Aralez Pharmaceuticals/ Merck, Lisata Therapeutics, and others which are currently developing drugs for the treatment of Peripheral Artery Disease (PAD).
3. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as incident cases, treatment costs, revenue generated, and market trends.
4. What is the key driver of the Peripheral Artery Disease (PAD) market?
The increase in diagnosed prevalent cases of Peripheral Artery Disease (PAD) and the launch of emerging therapies are attributed to be the key drivers for increasing the Peripheral Artery Disease (PAD) market.
5. What is the expected impact of emerging therapies or advancements in Peripheral Artery Disease (PAD) treatment on the market?
Introducing new therapies, advancements in diagnostic techniques, and innovations in treatment approaches can significantly impact the Peripheral Artery Disease (PAD) treatment market. Market forecast reports may provide analysis and predictions regarding the potential impact of these developments.
6. Does the report provide insights into the competitive landscape of the market?
The market forecast report may include information on the competitive landscape, profiling key market players, their product offerings, partnerships, and strategies, and helping stakeholders understand the competitive dynamics of the Peripheral Artery Disease (PAD) market.




_pulmonary-vascular-disease-(pvd).png&w=256&q=75)

