Progressive Pulmonary Fibrosis (PPF) Market
- The recent ATS 2024 International Conference featured extensive coverage of Progressive Pulmonary Fibrosis (PPF). Sessions included strategies to enhance care for patients with combined pulmonary fibrosis and emphysema, a study on the protective role of sex hormones in pulmonary fibrosis onset and progression, cellular genomics of bronchoalveolar lavage in understanding fibrosis mechanisms, and the potential of PEG-FUD as a probe targeting the pro-fibrotic disease phase.
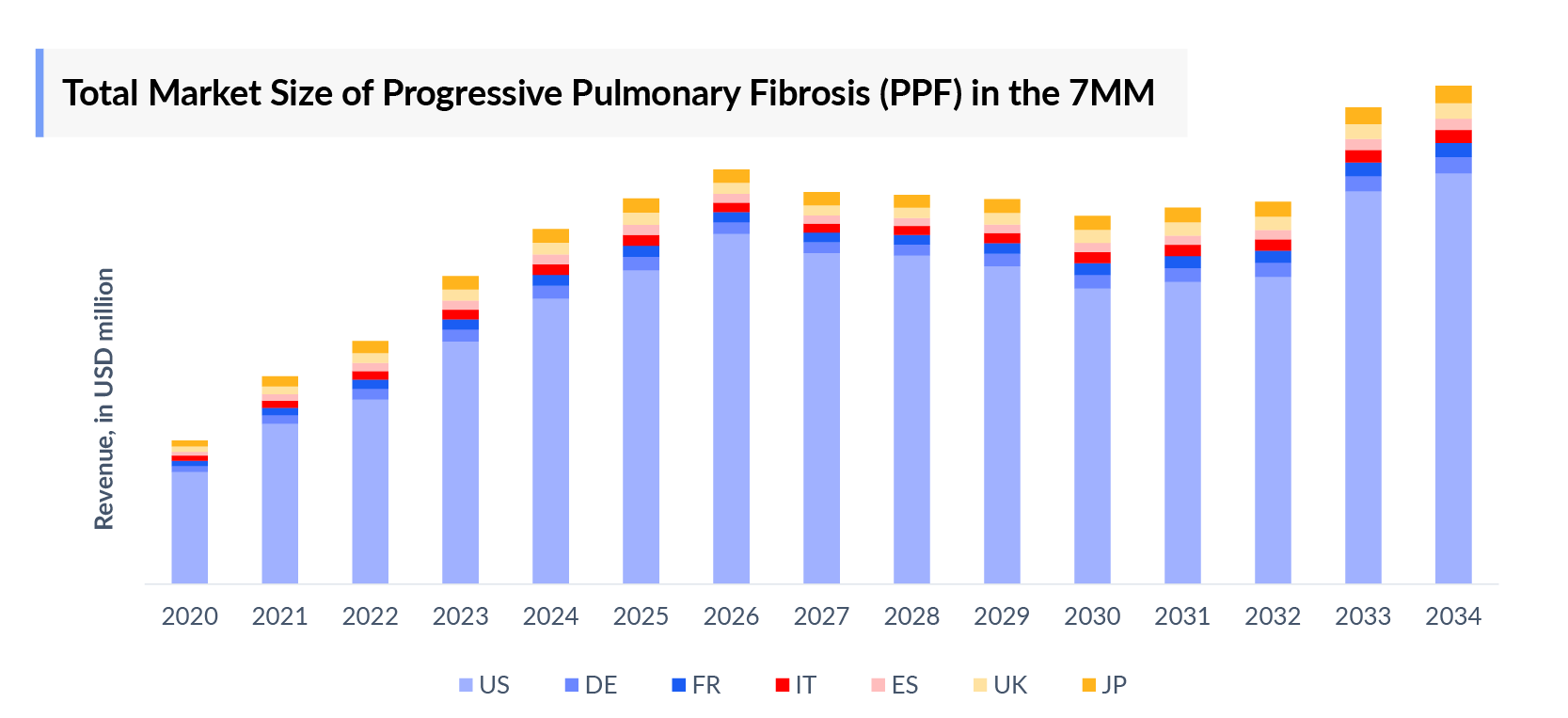
- In 2023, the market size of Progressive pulmonary fibrosis (PPF) was highest in the US among the 7MM, accounting for approximately USD 1,000 million which is further expected to increase by 2034.
- Various therapies are employed to treat Progressive Pulmonary Fibrosis (PPF), primarily falling into the categories of corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, tacrolimus, Ofev (nintedanib), and others such as cyclophosphamide and methotrexate. Among these, Ofev (nintedanib) had the highest market share in 2023, accounting for approximately USD 1,140 million in the 7MM, according to our analysis.
- The emerging drug BI 1015550 and is expected to launch in EU4 and the UK by 2027, and in Japan by 2028, which have the potential to reduce the disease burden of Progressive pulmonary fibrosis (PPF) in the forecasted years
DelveInsight’s “Progressive pulmonary fibrosis (PPF) Market Insights, Epidemiology, and Market Forecast – 2034” report deliver an in-depth understanding of the Progressive pulmonary fibrosis (PPF), historical and forecasted epidemiology as well as the Progressive pulmonary fibrosis (PPF) market trends in the United States, EU4 and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The Progressive pulmonary fibrosis (PPF) market report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Progressive pulmonary fibrosis (PPF) market size from 2020 to 2034. The Report also covers current Progressive pulmonary fibrosis (PPF) treatment practice, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
Progressive pulmonary fibrosis Treatment Market
Progressive pulmonary fibrosis Overview
Progressive pulmonary fibrosis consist of a diverse group of interstitial lung diseases (ILDs) characterized by a similar clinical phenotype of accelerated respiratory failure, frequent disease exacerbation and earlier mortality.
Interstitial lung disease is believed to be caused by long-term exposure to hazardous materials, such as asbestos or coal dust, or it can be caused by an auto-immune disease such as rheumatoid arthritis. Once lung scarring occurs, it is generally irreversible.
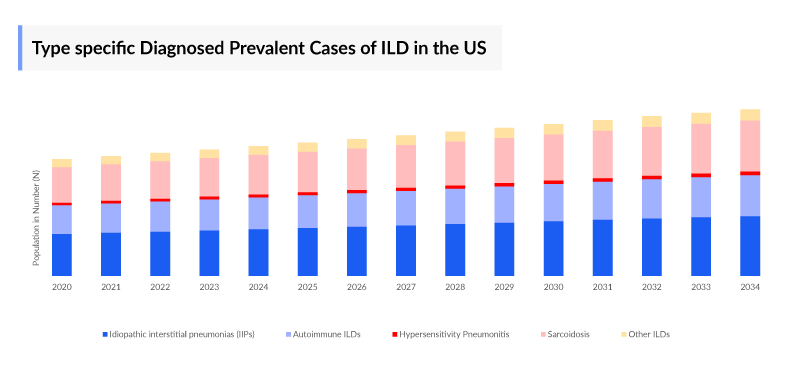
Progressive pulmonary fibrosis (PPF) are composed of Idiopathic Interstitial Pneumonias such as non-specific interstitial pneumonia and unclassifiable interstitial pneumonia, and inhalation lung diseases such as chronic hypersensitivity pneumonia, and connective tissue disease-associated ILD such as rheumatoid arthritis-related ILD and SSc-related ILD, and sarcoidosis and so on. Many interstitial lung diseases (ILDs) are characterized by chronic progressive fibrosis. The type-specific segmentation majorly includes, Idiopathic Interstitial Pneumonias (IIPs), Autoimmune ILDs, Hypersensitivity Pneumonitis, Sarcoidosis, and other ILDs.
Progressive pulmonary fibrosis (PPF) Diagnosis
Imaging tests such as X-ray and a high-resolution computed tomography (CT scan), is key to, and sometimes the first step in, the diagnosis of interstitial lung disease. The severity level of Progressive pulmonary fibrosis (PPF) is calculated on the basis of FVC score. However, identifying and determining the cause of interstitial lung disease can be challenging, as a large number of disorders fall into this broad category. Hence differential diagnosis is needed.
The differential diagnosis of Interstitial Lung Diseases requires a multidisciplinary approach, usually involving pulmonologist, radiologist, and pathologist. The evaluations include clinical presentation, specific history assessment, smoking status, lung function evaluation, serological test results, imaging and if required, lung biopsy.
Further details related to diagnosis are provided in the report...
Progressive pulmonary fibrosis (PPF) Treatment
Treatment for Interstitial Lung Diseases varies depending on the type of Interstitial Lung Disease diagnosed and the severity. Lung damage from Interstitial Lung Diseases is often irreversible and progressive, so treatment normally centers on relieving symptoms, improving quality of life and slowing the disease's progression. Medications, such as corticosteroids, can be used to decrease inflammation in the lungs.
Nintedanib (Ofev) is the only approved drug in the Progressive pulmonary fibrosis (PPF) treatment landscape. Along with Ofev the current market is mainly attributed to corticosteroids and immunosuppressant for treatment. Novel uses of antifibrotic therapies are emerging due to a paucity of evidence-based treatments for multiple Interstitial Lung Disease subtypes. The market is anticipates launch of several emerging therapies in the Progressive pulmonary fibrosis (PPF) treatment domain in the forecast period.
Further details related to treatment are provided in the report...
Progressive pulmonary fibrosis Epidemiology
As the market is derived using a patient-based model, the Progressive pulmonary fibrosis (PPF) epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by, Total Prevalent Cases of Interstitial Lung Disease (ILD), Total Diagnosed Prevalent Cases of Interstitial Lung Disease (ILD), Type-specific Diagnosed Prevalent Cases of Interstitial Lung Disease (ILD), Total Diagnosed Prevalent Cases of Progressive pulmonary fibrosis (PPF) in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
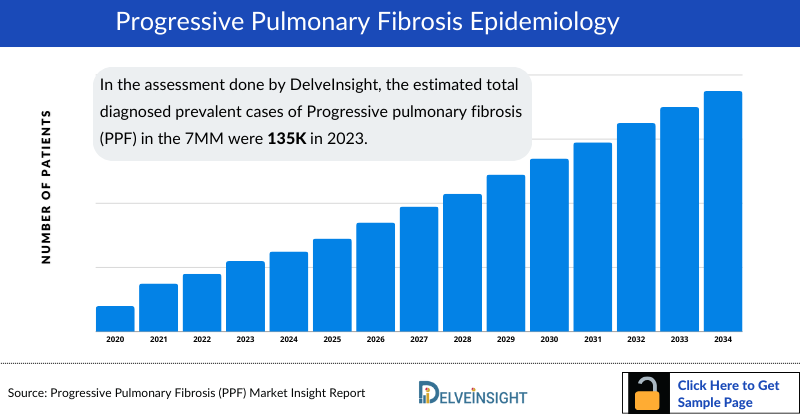
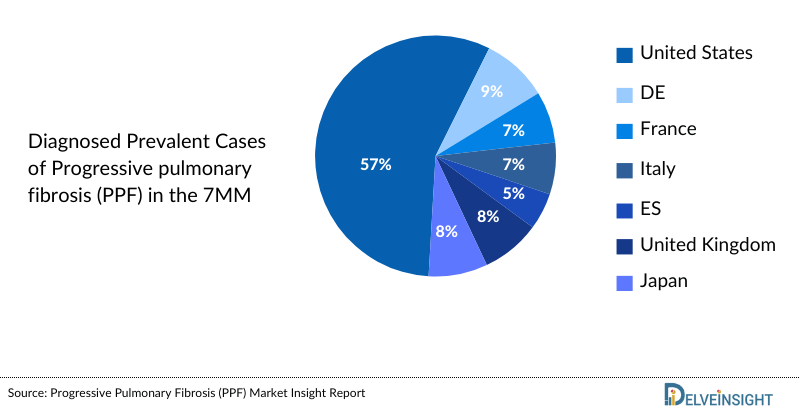
- In the assessment done by DelveInsight, the estimated total diagnosed prevalent cases of Progressive pulmonary fibrosis (PPF) in the 7MM were 135 thousand in 2023.
- The highest total diagnosed prevalent cases of Progressive pulmonary fibrosis (PPF) were accounted by the US in 2023 (77 thousand), which are expected to show a rise in the future.
- Among the European countries, Germany had the highest diagnosed prevalent cases of PPF with ~11 thousand cases, followed by the United Kingdom, which had prevalent population of 10 thousand in 2023. On the other hand, Spain had the lowest prevalent population (around 6 thousand cases).
- Japan had 10 thousand total diagnosed prevalent cases of Progressive pulmonary fibrosis (PPF) in 2023, accounting for approximately 8% in 7MM.
Progressive pulmonary fibrosis Drug Chapters
The drug chapter segment of the Progressive pulmonary fibrosis (PPF) report encloses a detailed analysis of Progressive pulmonary fibrosis (PPF) off-label drug and late-stage (Phase-III and Phase-II) pipeline drug. It also helps to understand the Progressive pulmonary fibrosis (PPF) clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Progressive pulmonary fibrosis Emerging Drugs
Nerandomilast (BI 1015550): Boehringer Ingelheim
BI 1015550 is an investigational, oral, phosphodiesterase 4B (PDE4B) inhibitor with combined antifibrotic and anti-inflammatory effects being developed by Boehringer Ingelheim. It has the potential to address both pulmonary fibrosis – an irreversible scarring of lung tissue that negatively impacts lung function – and inflammation associated with Progressive pulmonary fibrosis (PPF) (ILDs). BI 1015550 represents the first molecule in the class of PDE4B inhibitors that is being studied for IPF and other progressive fibrosing ILDs.
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
Nerandomilast (BI 1015550) |
PDE4B inhibitor |
Oral |
Boehringer Ingelheim |
III | |
|
XX |
LPA1 Antagonist |
XXX |
XXX |
III |
Note: Detailed emerging therapies assessment will be provided in the final report of Progressive pulmonary fibrosis (PPF)...
Progressive pulmonary fibrosis Market Outlook
Progressive pulmonary fibrosis (PPF) consist of a diverse group of interstitial lung diseases (ILDs) characterized by a similar clinical phenotype of accelerated respiratory failure, frequent disease exacerbation and earlier mortality.
ILD and its progressing forms, form a substantial proportion of disabling chronic lung disease leading to significant morbidity and mortality. From the current treatment market scenario, Nintedanib is the only approved treatment in the chronic fibrotic ILDs in the 7MM. Myriads of patients with PPFs are currently orphan of evidence-based treatments and are left with options of receiving corticosteroids and/or off-label immunosuppressive therapies with variable outcomes and are the mainstay of treatment in PPFs. These unmet treatment gaps accentuates the launch of emerging therapies.
In the upcoming treatment landscape, there are plethora of companies investigating agents for use in the PPF which includes Boehringer Ingelheim, Bristol-Myers Squibb, and others. There are many more pharma companies which are conducting clinical trials for therapies of PPF.
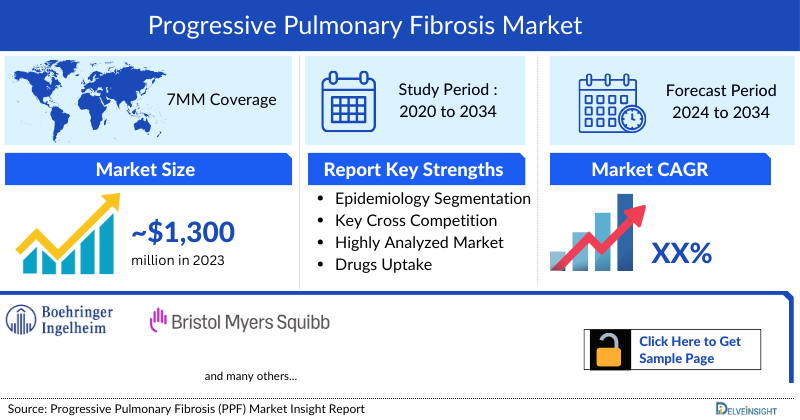
- The therapeutic market size of Progressive pulmonary fibrosis (PPF) in the 7MM was USD 1,300 million in 2023.
- The United States accounted for the highest market size of Progressive pulmonary fibrosis (PPF) approximately 79% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the EU countries, Germany had the highest market size with USD 50 million in 2023, while Spain had the lowest market size of Progressive pulmonary fibrosis (PPF) with USD 39 million in 2023.
- The market size for Progressive pulmonary fibrosis (PPF) in Japan was estimated to be USD 58 million in 2023, which accounts for 4% of the total 7MM market.
- With the expected launch of upcoming therapies, such as BI 1015550 the total market size of Progressive pulmonary fibrosis (PPF) is expected to show change in the upcoming years.
Progressive pulmonary fibrosis Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to launch in the market during 2020–2034. For example, BI 1015550 in the US is expected to be launched by 2026 with a peak shared of 5.6%. BI 1015550 is anticipated to take 6 years to peak with a fast uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Progressive pulmonary fibrosis (PPF) Pipeline Development Activities
The report provides insights into different Progressive pulmonary fibrosis clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Progressive pulmonary fibrosis (PPF) emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Progressive pulmonary fibrosis (PPF) evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from Johns Hopkins School of Medicine, Baltimore, MD, USA; Royal Brompton Hospital/Imperial College London, London, UK; Pulmonary Department, Mainz University Hospital, Mainz, Germany; Pulmonology Unit, GB Morgagni Hospital/University of Bologna, Forlì, Italy; Department of Respiratory Medicine and Allergy, Tosei General Hospital, Japan; and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Progressive pulmonary fibrosis (PPF) market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Progressive pulmonary fibrosis Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Progressive pulmonary fibrosis (PPF), explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Progressive pulmonary fibrosis (PPF) market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Progressive pulmonary fibrosis (PPF) market.
Progressive pulmonary fibrosis (PPF) Report Insights
- Progressive pulmonary fibrosis Patient Population
- Progressive pulmonary fibrosis Therapeutic Approaches
- Progressive pulmonary fibrosis (PPF) Pipeline Analysis
- Progressive pulmonary fibrosis (PPF) Market Size and Trends
- Existing and Future Market Opportunity
Progressive pulmonary fibrosis (PPF) Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Progressive pulmonary fibrosis (PPF) Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Progressive pulmonary fibrosis Drugs Uptake
- Key Progressive pulmonary fibrosis Market Forecast Assumptions
Progressive pulmonary fibrosis (PPF) Report Assessment
- Current Progressive pulmonary fibrosis Treatment Practices
- Progressive pulmonary fibrosis Unmet Needs
- Progressive pulmonary fibrosis Pipeline Product Profiles
- Progressive pulmonary fibrosis Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Progressive pulmonary fibrosis Market Drivers
- Progressive pulmonary fibrosis Market Barriers
Key Questions
Progressive pulmonary fibrosis Market Insights
- What was the Progressive pulmonary fibrosis (PPF) market share (%) distribution in 2020 and how it would look like in 2034?
- What would be the Progressive pulmonary fibrosis (PPF) total market size as well as market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Progressive pulmonary fibrosis (PPF) market size during the forecast period (2024–2034)?
- At what CAGR, the Progressive pulmonary fibrosis (PPF) market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the Progressive pulmonary fibrosis (PPF) market outlook across the 7MM during the forecast period (2024–2034)?
- What would be the Progressive pulmonary fibrosis (PPF) market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Progressive pulmonary fibrosis Epidemiology Insights
- What is the disease risk, burden, and unmet needs of Progressive pulmonary fibrosis (PPF)?
- What is the historical Progressive pulmonary fibrosis (PPF) patient population in the United States, EU5 (Germany, France, Italy, Spain, and the UK), and Japan?
- What would be the forecasted patient population of Progressive pulmonary fibrosis (PPF) at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Progressive pulmonary fibrosis (PPF)?
- Out of the above-mentioned countries, which country would have the highest prevalent population of Progressive pulmonary fibrosis (PPF) during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current Progressive pulmonary fibrosis Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Progressive pulmonary fibrosis (PPF) along with the approved therapy?
- What are the current treatment guidelines for the treatment of Progressive pulmonary fibrosis (PPF) in the US, Europe, And Japan?
- What are the Progressive pulmonary fibrosis (PPF) marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, and efficacy, etc.?
- How many companies are developing therapies for the treatment of Progressive pulmonary fibrosis (PPF)?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Progressive pulmonary fibrosis (PPF)?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Progressive pulmonary fibrosis (PPF) therapies?
- What are the recent therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Progressive pulmonary fibrosis (PPF) and their status?
- What are the key designations that have been granted for the emerging therapies for Progressive pulmonary fibrosis (PPF)?
- What are the 7MM historical and forecasted market of Progressive pulmonary fibrosis (PPF)?
Reasons to Buy Progressive pulmonary fibrosis Therapeutic Assessment Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Progressive pulmonary fibrosis (PPF) Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.
Frequently Asked Questions
1. What is the forecast period covered in the report?
The Progressive pulmonary fibrosis (PPF) Epidemiology and Market Insight report for the 7MM covers the forecast period from 2024 to 2034, providing a projection of market dynamics and trends during this timeframe.
2. Who are the key players in the Progressive pulmonary fibrosis (PPF) market?
The Progressive pulmonary fibrosis (PPF) market is quite robust. The major layers are Boehringer Ingelheim, Bristol-Myers Squibb, and others which are currently developing drugs for the treatment of Progressive pulmonary fibrosis (PPF).
3. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as incident cases, treatment costs, revenue generated, and market trends.
4. What is the key driver of the Progressive pulmonary fibrosis (PPF) market?
The increase in diagnosed prevalent cases of Progressive pulmonary fibrosis (PPF) and the launch of emerging therapies are attributed to be the key drivers for increasing Progressive pulmonary fibrosis (PPF) market.
5. What is the expected impact of emerging therapies or advancements in Progressive pulmonary fibrosis (PPF) treatment on the market?
Introducing new therapies, advancements in diagnostic techniques, and innovations in treatment approaches can significantly impact the Progressive pulmonary fibrosis (PPF) treatment market. Market forecast reports may provide analysis and predictions regarding the potential impact of these developments.
6. Does the report provide insights into the competitive landscape of the market?
The market forecast report may include information on the competitive landscape, profiling key market players, their product offerings, partnerships, and strategies, and helping stakeholders understand the competitive dynamics of the Progressive pulmonary fibrosis (PPF) market.