Recurrent Respiratory Papillomatosis Market Summary
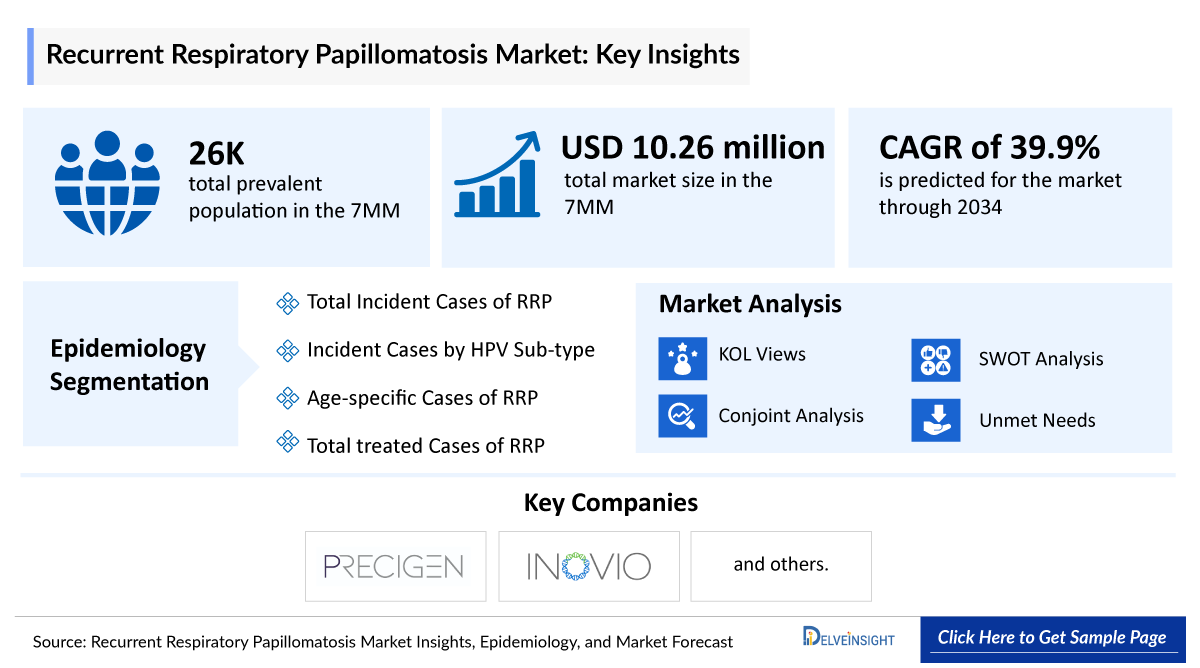
- The Recurrent Respiratory Papillomatosis market in the 7MM was valued at approximately USD 10.26 million in 2023 and is projected to grow substantially, with a strong CAGR of 39.9% expected through 2034.
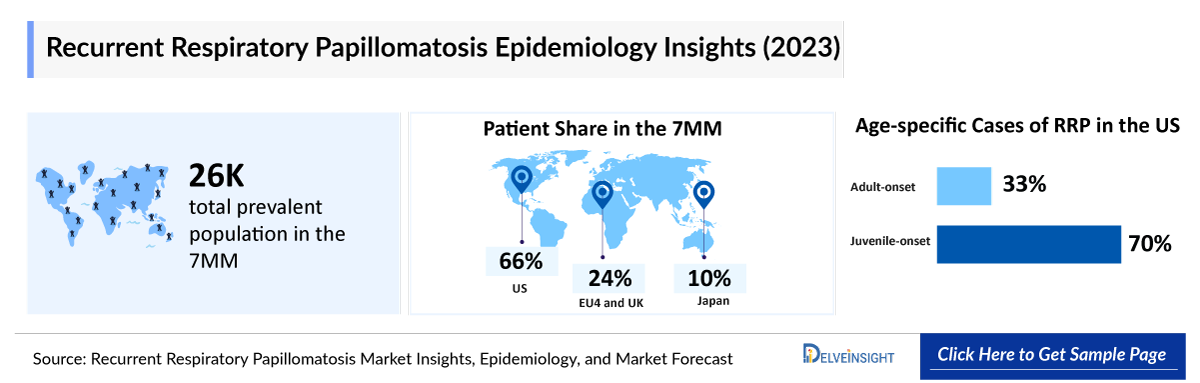
- Recurrent Respiratory Papillomatosis is a rare but challenging disease characterized by the formation of benign tumors, or papillomas, in the respiratory tract, primarily affecting the larynx, trachea, and bronchi. The primary cause of RRP is infection with HPV, particularly HPV types 6 and 11. In 2023, the prevalent population of RRP in the 7MM is estimated to be around 25,700.
- In 2023, the US represented the largest share of the diagnosed RRP population within the 7MM, accounting for 66% of the total cases.
- RRP represents a growing health concern in the United States, with its prevalence rising over decades. DelveInsight estimates indicate that among the different age groups in 2023, the highest number of cases were observed in those aged 18 years and above (~15,800), followed by those aged 0-8 years (~660), and the least number of cases is in the 9-17 years age group (~500).
- In 2023, Japan reported approximately 2,500 diagnosed prevalent cases of RRP among which ~2,280 cases were associated with HPV 6 and/or HPV 11, while ~200 cases were linked to other high-risk subtypes such as HPV-16, 18, 31, 33, 39, and other factors. This data highlights that the majority of RRP cases in Japan are predominantly driven by HPV 6 and 11, with a significantly smaller proportion attributable to other high-risk HPV subtypes and additional factors. The disparity in case numbers underscores the critical role of HPV 6 and 11 in the etiology of RRP within the Japanese population.
- In 2023, the US led the 7MM in market size for RRP, reaching approximately USD 7.8 million, which constituted about 76% of the total market share within these regions. This dominant position is projected to grow further, with an anticipated significant Compound Annual Growth Rate (CAGR) of 40.2% by 2034, reflecting strong market expansion driven by increasing demand and advancements in RRP treatment in the US.
- The RRP market is driven by increasing prevalence, advancements in treatment options, and expanded awareness of the disease. Key barriers include limited availability of effective therapies, high treatment costs, and challenges in early diagnosis. Additionally, the rarity of the condition and varying regulatory environments across regions pose significant hurdles to market growth and patient access to treatment.
- In August 2024, Precigen revealed a strategic realignment to focus on the potential commercialization of PRGN-2012. This shift involves reducing its workforce by 20% and suspending all preclinical programs. The move underscores the company's commitment to advancing PRGN-2012 while reallocating resources to support its commercial prospects.
- In December 2024, Precigen, Inc. announced the completion of its biologics license application (BLA) submission to the FDA for PRGN-2012 (zopapogene imadenovec†) to treat adult Recurrent Respiratory Papillomatosis.
- In August 2024, Inovio Pharmaceuticals reported progress towards submitting its BLA under the FDA's Accelerated Approval pathway, following a pre-BLA meeting and advancing BLA modules. Despite these developments, a manufacturing issue with the CELLECTRA device's disposable component has delayed the submission to mid-2025. Inovio is actively addressing this challenge and is anticipated to report more details in their next quarterly report.
- In June 2024, encouraging results from the Phase I/II pivotal study of PRGN-2012, an investigational off-the-shelf AdenoVerse gene therapy for RRP, were disclosed and presented during a late-breaking oral session at the 2024 American Society of Clinical Oncology (ASCO) annual meeting.
- The total market size of RRP is anticipated to upsurge during the forecast period due to the expected entry of emerging therapies that includes INO-3107, PRGN-2012, and others.
Key Factors Driving Recurrent Respiratory Papillomatosis (RRP) Market
Rising Recurrent Respiratory Papillomatosis Patient Pool
The diagnosed prevalence of Recurrent Respiratory Papillomatosis (RRP) in the 7MM is projected to rise gradually from nearly 25,700 cases in 2023 as awareness, diagnostic advancements, and better screening practices improve disease identification. The United States represents the largest diagnosed patient share, accounting for about 66% of the cases, highlighting a substantial treatment opportunity.
Advancements in Recurrent Respiratory Papillomatosis Therapeutics
While surgical intervention remains the cornerstone of RRP management, the therapeutic landscape is witnessing innovation with pharmacological options gaining traction. Bevacizumab has emerged as a promising treatment in severe RRP cases, reducing recurrence and surgical frequency. Meanwhile, antiviral therapies such as cidofovir, ribavirin, and acyclovir, along with novel approaches like mTOR inhibitors, are being increasingly explored, broadening the treatment paradigm for RRP.
Preventive and Vaccine-Based Approaches in Recurrent Respiratory Papillomatosis
The approval and adoption of HPV vaccines such as Gardasil, which targets HPV types 6 and 11—the main causative agents of RRP—has created new preventive opportunities. Vaccination not only reduces the risk of HPV-driven papilloma formation but also demonstrates potential in reducing disease recurrence among already diagnosed patients, offering a major breakthrough in long-term management strategies.
Robust Clinical Trial Pipeline in Recurrent Respiratory Papillomatosis
A wave of emerging therapies for Recurrent Respiratory Papillomatosis is shaping the future treatment landscape. Key candidates such as PRGN-2012 (Precigen, Inc.) and INO-3107 (Inovio Pharmaceuticals) are being evaluated in clinical trials and hold the potential to significantly alter disease outcomes. The ongoing research and development efforts by multiple companies reflect strong industry interest and bring hope for more effective and durable treatment options.
DelveInsight’s “Recurrent Respiratory Papillomatosis Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of the RRP, historical and forecasted epidemiology as well as the Recurrent Respiratory Papillomatosis therapeutics market trends in the United States, EU4 and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The RRP market report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM RRP market size from 2020 to 2034. The Report also covers current RRP treatment practice, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Recurrent Respiratory Papillomatosis Treatment Market
Recurrent Respiratory Papillomatosis Overview
RRP is a rare, chronic disease characterized by the growth of benign tumors, known as papillomas, in the respiratory tract, particularly affecting the larynx and vocal cords. These growths, caused by human papillomavirus (HPV) types 6 and 11, can obstruct the airway, leading to voice changes, breathing difficulties, and recurring surgeries. RRP manifests in two forms: juvenile-onset, typically diagnosed in early childhood, and adult-onset, appearing later in life. Despite its benign nature, RRP is challenging to manage due to its recurrent nature and the lack of a definitive cure.
Recurrent Respiratory Papillomatosis Diagnosis
RRP diagnosis involves a combination of clinical evaluation, endoscopic examination, and histopathological analysis. A laryngoscopy or bronchoscopy is commonly used to visualize and assess the presence of papillomas in the respiratory tract. Biopsy samples are analyzed to confirm HPV infection and differentiate RRP from other conditions.
Diagnosing RRP can be challenging due to its variable presentation and overlap with other respiratory disorders. Additionally, the disease’s intermittent nature and the potential for misdiagnosis or delayed diagnosis further complicate accurate identification, necessitating a high index of suspicion and comprehensive evaluation by specialized clinicians.
Further details related to diagnosis are provided in the report…
Recurrent Respiratory Papillomatosis Treatment
Treatment of RRP focuses on symptom management and complication prevention, as no definitive cure exists. The primary approach involves surgical removal of papillomas to clear the airway and restore voice function. Techniques such as laser ablation, microdebrider removal, and cold knife excision are chosen based on papilloma size, location, and number. Due to the recurrent nature of RRP, multiple surgeries are often necessary over time. Additionally, antiviral therapies, including interferon-alpha, may be utilized to mitigate papilloma recurrence. While these treatments aim to manage symptoms and reduce the frequency of surgery, they do not address the underlying cause of papilloma growth, highlighting the need for more effective long-term solutions.
Despite available treatments for RRP significant unmet needs remain. Current therapies primarily focus on managing symptoms and controlling papilloma growth, with no definitive cure. Surgical interventions, while effective, often require frequent procedures due to the recurrent nature of the disease, leading to substantial patient burden and healthcare costs. Antiviral and immunotherapeutic approaches offer some promise but are not universally effective and may not address the underlying cause of papilloma recurrence.
There is a critical need for more effective, long-lasting treatments that reduce recurrence rates, improve patient quality of life, and minimize the need for repeated surgeries. Additionally, advancements in early diagnosis and targeted therapies could enhance treatment outcomes for RRP patients.
Further details related to treatment are provided in the report…
Recurrent Respiratory Papillomatosis Epidemiology
As the market is derived using the patient-based model, the Recurrent Respiratory Papillomatosis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Diagnosed Prevalent Cases of RRP, Age-specific Diagnosed Prevalent Cases of RRP, and Etiology-specific Diagnosed Prevalent Cases of RRP in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan, from 2020 to 2034.
- The total diagnosed prevalent cases of RRP in the US comprised approximately 16,980 in 2023 and are projected to increase during the forecast period.
- As per DelveInsight’s estimates, the US alone accounts the highest number of total diagnosed prevalent cases, of RRP in the 7MM, followed by EU4 and the UK and Japan, contributing to 24% and 10% of all RRP cases respectively.
- In 2023, Japan reported the highest number of RRP cases among adults aged 18 and older (~2,400), followed by adolescents aged 9-17 years (~50). The smallest number of cases was in children aged 0-8 years, totaling ~25.
- In 2023, the EU4 and the UK reported a total of ~6,240 diagnosed prevalent cases of RRP, with approximately 5,740 cases associated with HPV 6 and/or HPV 11, and ~500 cases linked to other high-risk subtypes like HPV-16, 18, 31, 33, and 39. The high number of cases related to HPV 6 and 11 reflects the predominant role these strains play in RRP, as they are the primary etiological agents. The relatively lower number of cases associated with other high-risk HPV subtypes indicates their less frequent but still significant contribution to RRP, underscoring the need for targeted interventions and monitoring of HPV-related factors in managing the disease.
- In 2023, Germany reported the highest number of diagnosed prevalent RRP cases in the EU4 and the UK, totaling nearly 1,684. France followed with approximately 1,370 cases, Italy had ~1,220, the UK reported ~1,022, and Spain had the fewest with ~944 cases. This distribution highlights varying disease burden across European countries.
Stay ahead with insights on Recurrent Respiratory Papillomatosis prevalence and patient population projections.
Recurrent Respiratory Papillomatosis Recent Developments
- In Feb 2025, Precigen, Inc. announced that the FDA granted priority review to its biologics license application (BLA) for PRGN-2012 (zopapogene imadenovec), an investigational AdenoVerse gene therapy for treating adults with recurrent respiratory papillomatosis (RRP).
- In December 2024, Precigen, Inc. announced the completion of its biologics license application (BLA) submission to the FDA for PRGN-2012 (zopapogene imadenovec†) to treat adult Recurrent Respiratory Papillomatosis.
Recurrent Respiratory Papillomatosis Drug Chapters
The drug chapter segment of the RRP report encloses a detailed analysis of RRP off-label drugs and late-stage (Phase-III and Phase-I/II) pipeline drugs. It also helps to understand the RRP clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Emerging Recurrent Respiratory Papillomatosis Drugs
PRGN-2012: Precigen, Inc.
PRGN-2012 is a pioneering gene therapy for Recurrent Respiratory Papillomatosis, utilizing Precigen’s advanced gorilla adenovector technology within the AdenoVerse platform. This first-in-class therapy enhances immune responses by targeting HPV types 6 and 11 through an optimized antigen design, administered via subcutaneous injection. Currently in Phase III, PRGN-2012 leverages high-capacity, low-seroprevalence gorilla adenovectors for effective gene delivery and immune modulation. The FDA has highlighted the importance of the Phase I/II trial for potential accelerated approval.
PRGN-2012 has received Breakthrough Therapy and Orphan Drug designations from both the FDA and the European Commission, underscoring its significance.
As of August 2024, Precigen expects to begin the rolling BLA submission for PRGN-2012 under the accelerated approval pathway in the latter half of 2024. The company has also initiated participant enrollment for the confirmatory clinical trial (NCT06538480) of PRGN-2012.
In June 2024, Precigen and the RRP Foundation launched the inaugural RRP Awareness Day. This collaborative event brought together patients, caregivers, clinicians, and government officials to enhance awareness and understanding of RRP.
INO-3107: Inovio Pharmaceuticals
INO-3107, developed by Inovio Pharmaceuticals, is a DNA-based therapy aimed at treating RRP using a targeted approach. This investigational treatment employs Inovio's proprietary technology to deliver a therapeutic gene encoding HPV E6 and E7 antigens directly into patient cells. By stimulating a strong immune response against these HPV proteins, INO-3107 seeks to diminish papilloma formation and recurrence. Utilizing plasmid DNA and electroporation to boost cellular uptake and antigen expression, the therapy aims to enhance immune recognition and elimination of the papillomavirus. INO-3107 aims to activate T cells to target HPV-6 and HPV-11, which could help reduce papilloma growth.
In July 2024, INO-3107 received the Innovation Passport designation from the UK Government’s Innovative Licensing and Access Pathway. This accolade underscores INO-3107's groundbreaking potential and facilitates its progress through the regulatory process, enhancing its prospects for advancing medical innovation and improving patient outcomes.
In July 2024, Inovio received the Advanced Therapy Medicinal Product (ATMP) Certificate from the European Medicines Agency (EMA) for INO-3107. This certification highlights the strength of the quality and non-clinical data backing INO-3107, representing a crucial step forward in its progression through the European regulatory process.
Recurrent Respiratory Papillomatosis Market Outlook
The treatment landscape for RRP has expanded significantly, incorporating a range of pharmacological therapies to manage the virus effectively. Surgical removal of tumors from the larynx or airway remains the primary treatment for RRP, aiming to excise papillomas while preserving healthy tissue. Beyond surgery, pharmacological treatments are explored, including off-label options. Bevacizumab, an anti-angiogenic agent, has shown promise in severe cases by reducing surgery frequency and improving disease management. Administered intravenously at intervals of 3–4 weeks, its dosage is adjusted based on response. Interferon therapy, once a key RRP treatment, is now less commonly used due to side effects and limited efficacy, though it may be considered for refractory cases. Antiviral agents like acyclovir, ribavirin, and cidofovir play roles in managing RRP, with cidofovir specifically targeting HPV but facing safety concerns. mTOR inhibitors, such as sirolimus, are under investigation for their potential to control papilloma growth. Additionally, the HPV vaccine Gardasil, offers prevention against HPV types 6 and 11 and may reduce RRP recurrence.
The launch of emerging therapies, such as PRGN-2012, INO-3107, and others are expected to impact the market positively. The approval of these therapies could significantly impact market dynamics, although their success rates remain uncertain.
- The market size of RRP in the 7MM was nearly USD 10.26 million in 2023, which is further anticipated to increase during the forecast period with a significant compound annual growth rate (CAGR) of 39.9%.
- The EU4 and the UK accounted for the approximately 16% of the 7MM market size, amounting the value of USD ~1.66 million market size of RRP approximately in 2023.
- Among the EU countries, Germany had the highest market size with nearly USD 0.45 million in 2023, while Spain had the lowest market size for RRP with about USD 0.25 million in 2023.
- In 2023, Japan accounted for 8% of the total 7MM market, with a market value of approximately USD 0.77 million.
Recurrent Respiratory Papillomatosis Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to launch in the market during 2020–2034. For example, PRGN-2012 in the US is expected to be launched by 2025 with a peak share of 50.0%. INO-3107 is anticipated to take 7 years to peak with a slow-medium uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Recurrent Respiratory Papillomatosis Pipeline Development Activities
The report provides insights into different Recurrent Respiratory Papillomatosis clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for RRP emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on RRP evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from University of Chicago Medicine, US; Massachusetts General Hospital, US; University Medical Center Göttingen, Göttingen, Germany; Strasbourg University Hospital, Strasbourg, France; University of Genoa, Genova, Italy; University of Barcelona, Spain; Sandwell and West Birmingham Hospitals NHS Trust, UK; Osaka University Graduate School of Medicine, Suita, Japan, and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or RRP market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Recurrent Respiratory Papillomatosis Market Forecast Report
- The report covers a segment of key events, an executive summary, descriptive overview of RRP, explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the RRP market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM RRP market.
Recurrent Respiratory Papillomatosis Report Insights
- Recurrent Respiratory Papillomatosis Patient Population
- Recurrent Respiratory Papillomatosis Therapeutic Approaches
- Recurrent Respiratory Papillomatosis Pipeline Analysis
- Recurrent Respiratory Papillomatosis Market Size and Trends
- Existing and Future Market Opportunity
Recurrent Respiratory Papillomatosis Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- RRP Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
Drugs Uptake and Key Market Forecast Assumptions
- Recurrent Respiratory Papillomatosis Report Assessment
- Current Recurrent Respiratory Papillomatosis Treatment Practices
- Recurrent Respiratory Papillomatosis Unmet Needs
- Recurrent Respiratory Papillomatosis Pipeline Product Profiles
- Recurrent Respiratory Papillomatosis Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Recurrent Respiratory Papillomatosis Market Drivers
- Recurrent Respiratory Papillomatosis Market Barriers
Key Questions Answered In The Recurrent Respiratory Papillomatosis Market Report:
Recurrent Respiratory Papillomatosis Market Insights
- What was the RRP market share (%) distribution in 2020 and how it would look like in 2034?
- What would be the RRP total market size as well as market size by therapies across the 7MM during the forecast period (2024–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest RRP market size during the forecast period (2024–2034)?
- At what CAGR, the RRP market is expected to grow at the 7MM level during the forecast period (2024–2034)?
- What would be the RRP market outlook across the 7MM during the forecast period (2024–2034)?
- What would be the RRP market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Recurrent Respiratory Papillomatosis Epidemiology Insights
- What is the disease risk, burden, and unmet needs of RRP?
- What is the historical RRP patient population in the United States, EU5 (Germany, France, Italy, Spain, and the UK), and Japan?
- What would be the forecasted patient population of RRP at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to RRP?
- Out of the above-mentioned countries, which country would have the highest prevalent population of RRP during the forecast period (2024–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024–2034)?
Current Recurrent Respiratory Papillomatosis Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of RRP along with the approved therapy?
- What are the current treatment guidelines for the treatment of RRP in the US, Europe, And Japan?
- What are the RRP marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, and efficacy, etc.?
- How many companies are developing therapies for the treatment of RRP?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of RRP?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the RRP therapies?
- What are the recent therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for RRP and their status?
- What are the key designations that have been granted for the emerging therapies for RRP?
- What are the 7MM historical and forecasted market of RRP?
Reasons to Buy Recurrent Respiratory Papillomatosis Market Forecast Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the RRP Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.
Frequently Asked Questions
1. What is the forecast period covered in the report?
The RRP Epidemiology and Market Insight report for the 7MM covers the forecast period from 2024 to 2034, providing a projection of market dynamics and trends during this timeframe.
2. Who are the key players in the RRP market?
The RRP emerging market has few products. The major layers are Precigen Inc., Inovio Pharmaceuticals, and others which are currently developing drugs for the treatment of RRP.
3. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as prevalent cases, treatment costs, revenue generated, and market trends.
4. What is the key driver of the RRP market?
The increase in diagnosed prevalent cases of RRP and the launch of emerging therapies are attributed to be the key drivers for increasing the RRP market.
5. What is the expected impact of emerging therapies or advancements in RRP treatment on the market?
Introducing new therapies, advancements in diagnostic techniques, and innovations in treatment approaches can significantly impact the RRP treatment market. Market forecast reports may provide analysis and predictions regarding the potential impact of these developments.
6. Does the report provide insights into the competitive landscape of the market?
The market forecast report may include information on the competitive landscape, profiling key market players, their product offerings, partnerships, and strategies, and helping stakeholders understand the competitive dynamics of the RRP market.

.png)

