Relapsing-Remitting Multiple Sclerosis Pipeline
DelveInsight’s, “Relapsing-Remitting Multiple Sclerosis - Pipeline Insight, 2025” report provides comprehensive insights about 20+ companies and 22+ pipeline drugs in Relapsing-Remitting Multiple Sclerosis pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Geography Covered
- Global coverage
Relapsing-Remitting Multiple Sclerosis: Understanding
Relapsing-Remitting Multiple Sclerosis: Overview
Relapsing-Remitting Multiple Sclerosis (RRMS) is the most common form of multiple sclerosis, characterized by clearly defined episodes of neurological symptoms (relapses) followed by periods of partial or complete recovery (remissions). During relapses, inflammation leads to demyelination and nerve damage in the central nervous system, resulting in symptoms such as visual disturbances, muscle weakness, balance issues, and fatigue. Although remissions may bring symptom improvement, some damage can accumulate over time. RRMS typically begins in young adulthood and can progress to more severe forms if not managed. Early diagnosis and treatment with disease-modifying therapies are crucial to reduce relapse frequency and delay disability progression.
Relapsing-Remitting Multiple Sclerosis (RRMS) is marked by periods of neurological symptoms (relapses) followed by recovery (remissions). Common symptoms during a relapse include visual disturbances, muscle weakness, numbness, fatigue, balance problems, spasticity, and cognitive difficulties. These symptoms can vary widely depending on the areas of the central nervous system affected. While symptoms may improve or resolve during remissions, some long-term neurological damage can accumulate over time, making early diagnosis and treatment crucial for managing the condition. Over time, some patients may transition to a secondary progressive form, where symptoms steadily worsen without distinct relapses. Disease-modifying therapies aim to reduce relapse frequency and slow disease progression.
The pathophysiology of Relapsing-Remitting Multiple Sclerosis (RRMS) involves an immune-mediated attack on the central nervous system (CNS), specifically targeting the myelin sheath that surrounds and protects nerve fibers. This process is thought to be initiated by abnormal activation of the immune system, where T cells and other immune cells cross the blood-brain barrier and recognize myelin as foreign, leading to inflammation and demyelination. The loss of myelin disrupts normal nerve signal transmission, resulting in the neurological symptoms seen during relapses. In the remission phase, the body attempts to repair the damaged myelin, but the repair process is incomplete, and over time, axonal damage may occur, contributing to disease progression. The cycles of relapse and remission are driven by ongoing immune dysregulation and inflammatory responses, and although remissions provide symptom relief, cumulative damage to the nervous system can lead to permanent disability.
The treatment and management of Relapsing-Remitting Multiple Sclerosis (RRMS) focus on controlling relapses, slowing disease progression, and improving the quality of life. Disease-modifying therapies (DMTs) are the cornerstone of treatment, aimed at reducing relapse frequency, preventing new lesions, and slowing disability progression. Common DMTs include interferon beta, glatiramer acetate, oral medications like fingolimod, and newer biologics such as natalizumab and ocrelizumab. During relapses, high-dose corticosteroids like methylprednisolone are often used to reduce inflammation and shorten the duration of symptoms. Symptomatic treatments also play a role, addressing fatigue, spasticity, pain, bladder dysfunction, and cognitive issues with medications and physical therapy. Lifestyle modifications, including regular exercise, a balanced diet, and stress management, are crucial for overall well-being. Regular monitoring and follow-up with neurologists help adjust treatments based on disease progression and response.
"Relapsing-Remitting Multiple Sclerosis- Pipeline Insight, 2025" report by DelveInsight outlays comprehensive insights of present scenario and growth prospects across the indication. A detailed picture of the Relapsing-Remitting Multiple Sclerosis pipeline landscape is provided which includes the disease overview and Relapsing-Remitting Multiple Sclerosis treatment guidelines. The assessment part of the report embraces, in depth Relapsing-Remitting Multiple Sclerosis commercial assessment and clinical assessment of the pipeline products under development. In the report, detailed description of the drug is given which includes mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, Relapsing-Remitting Multiple Sclerosis collaborations, licensing, mergers and acquisition, funding, designations and other product related details.
Report Highlights
- The companies and academics are working to assess challenges and seek opportunities that could influence Relapsing-Remitting Multiple Sclerosis R&D. The therapies under development are focused on novel approaches to treat/improve Relapsing-Remitting Multiple Sclerosis.
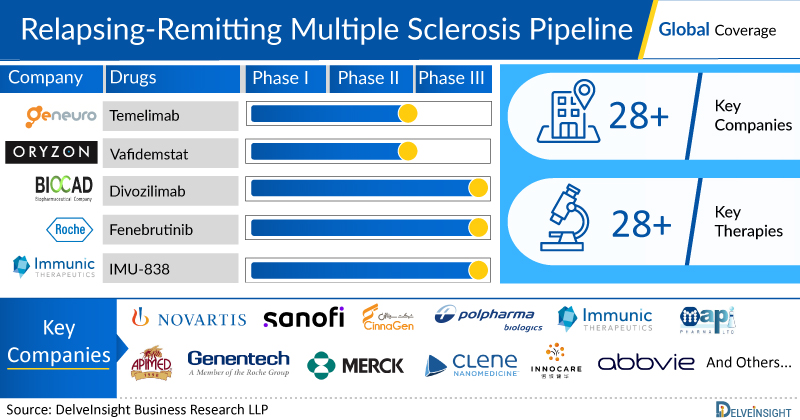
Relapsing-Remitting Multiple Sclerosis Emerging Drugs Chapters
This segment of the Relapsing-Remitting Multiple Sclerosis report encloses its detailed analysis of various drugs in different stages of clinical development, including Phase III, II, I, Preclinical and Discovery. It also helps to understand clinical trial details, expressive pharmacological action, agreements and collaborations, and the latest news and press releases.
Relapsing-Remitting Multiple Sclerosis Emerging Drugs
- IMU-838: Immunic AG
Vidofludimus calcium is an orally administered investigational small molecule drug being developed for chronic inflammatory and autoimmune diseases, currently in late-stage clinical trials for multiple sclerosis (MS). Uniquely, vidofludimus calcium’s first-in-class, dual mode of action combines neuroprotective, anti-inflammatory and anti-viral effects to target the complex pathophysiology of MS. As a selective immune modulator, it activates the neuroprotective transcription factor, nuclear receptor-related 1 (Nurr1), which provides direct and indirect neuroprotective effects. Additionally, vidofludimus calcium achieves anti-inflammatory and anti-viral effects through highly selective inhibition of the enzyme dihydroorotate dehydrogenase (DHODH). Currently, the drug is in Phase III stage of its development for the treatment of Relapsing-Remitting Multiple Sclerosis.
- IMCY-0141: Imcyse SA
IMCY-0141 Imotope™ is designed based on MOG (Myelin Oligodendrocyte Glycoprotein) with the aim to halt the progression of multiple sclerosis (MS) by stopping the body’s immune system from attacking the central nervous system and disrupting undesirable autoimmune responses that drive the destruction of the myelin sheath protecting the nerves. IMCY-0141 has shown promising results in several MS preclinical models, demonstrating an immune response that supports the proposed mode of action and inducing a memory response so that the treatment effect is long-lasting and requires less frequent dosing regimens. Also, if treatment is begun early enough, it has the potential to allow patients to live with minimal impact from the disease. Currently, the drug is in Phase I/II stage of its development for the treatment of Relapsing-Remitting Multiple Sclerosis.
- ANK-700: Anokion SA
ANK-700 is an investigational therapy developed by Anokion for the treatment of Relapsing-Remitting Multiple Sclerosis (RRMS). It employs a novel approach known as an ""inverse vaccine,"" aiming to re-educate the immune system to recognize specific myelin proteins as ""self,"" thereby preventing autoimmune attacks on the central nervous system. This strategy seeks to reduce neuroinflammation while preserving overall immune function. Anokion's approach utilizes its proprietary immune tolerance platform, which targets natural pathways in the liver to restore immune tolerance. This liver-targeted antigen strategy effectively expands antigen-specific regulatory T-cells in vivo, offering therapeutic potential across various inflammatory conditions, including multiple sclerosis. ANK-700's innovative mechanism and encouraging early clinical data position it as a promising candidate for future disease-modifying therapies in RRMS. Currently, the drug is in Phase I stage of its development for the treatment of Relapsing-Remitting Multiple Sclerosis.
Further product details are provided in the report……..
Relapsing-Remitting Multiple Sclerosis: Therapeutic Assessment
This segment of the report provides insights about the different Relapsing-Remitting Multiple Sclerosis drugs segregated based on following parameters that define the scope of the report, such as:
Major Players in Relapsing-Remitting Multiple Sclerosis
There are approx. 20+ key companies which are developing the therapies for Relapsing-Remitting Multiple Sclerosis. The companies which have their Relapsing-Remitting Multiple Sclerosis drug candidates in the most advanced stage, i.e. Phase III include, Immunic AG.
Phases
DelveInsight’s report covers around 22+ products under different phases of clinical development like
- Late stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
Route of Administration
Relapsing-Remitting Multiple Sclerosis pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
- Oral
- Intravenous
- Subcutaneous
- Parenteral
- Topical
Molecule Type
Products have been categorized under various Molecule types such as
- Recombinant fusion proteins
- Small molecule
- Monoclonal antibody
- Peptide
- Polymer
- Gene therapy
Product Type
Drugs have been categorized under various product types like Mono, Combination and Mono/Combination.
Relapsing-Remitting Multiple Sclerosis: Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, II, I, preclinical and discovery stage. It also analyses Relapsing-Remitting Multiple Sclerosis therapeutic drugs key players involved in developing key drugs.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing along with a thorough therapeutic assessment of emerging Relapsing-Remitting Multiple Sclerosis drugs.
Relapsing-Remitting Multiple Sclerosis Report Insights
- Relapsing-Remitting Multiple Sclerosis Pipeline Analysis
- Therapeutic Assessment
- Unmet Needs
- Impact of Drugs
Relapsing-Remitting Multiple Sclerosis Report Assessment
- Pipeline Product Profiles
- Therapeutic Assessment
- Pipeline Assessment
- Inactive drugs assessment
- Unmet Needs
Key Questions
Current Treatment Scenario and Emerging Therapies:
- How many companies are developing Relapsing-Remitting Multiple Sclerosis drugs?
- How many Relapsing-Remitting Multiple Sclerosis drugs are developed by each company?
- How many emerging drugs are in mid-stage, and late-stage of development for the treatment of Relapsing-Remitting Multiple Sclerosis?
- What are the key collaborations (Industry–Industry, Industry–Academia), Mergers and acquisitions, licensing activities related to the Relapsing-Remitting Multiple Sclerosis therapeutics?
- What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Relapsing-Remitting Multiple Sclerosis and their status?
- What are the key designations that have been granted to the emerging drugs?


