Seasonal Allergic Rhinitis Market Summary
Seasonal Allergic Rhinitis Market Insights and Epidemiological Forecast
- The Seasonal Allergic Rhinitis Market is expected to experience steady growth during the forecast period (2024–2034). Factors driving this growth include the increasing prevalence of allergies, growing awareness and diagnosis of the condition, and advancements in treatment options.
- DelveInsight’s analyst estimated that there had been an increase in Seasonal Allergic Rhinitis occurrences in recent years, which may be attributed to several factors such as climate change, air pollution, urbanization, and changes in allergen sensitization.
- The leading Seasonal Allergic Rhinitis Companies such as Regeneron Pharmaceuticals, Revolo Biotherapeutics, Allergy Therapeutics, Emergo Therapeutics, and others.
Request for Sample Page of the "Seasonal Allergic Rhinitis Drugs Market"
Key Factors Driving the Seasonal Allergic Rhinitis Market Growth
-
Rising Prevalence of Allergic Disorders
The increasing global incidence of allergic conditions such as seasonal allergic rhinitis—triggered by pollen, grasses, and mold spores—is a major growth driver. Environmental changes and lifestyle factors are contributing to higher allergy rates across age groups.
-
Environmental and Climate Change
Shifts in climate patterns, longer pollen seasons, and increased air pollution levels are intensifying allergen exposure. These environmental changes exacerbate symptoms and lead to higher diagnosis and treatment rates.
-
Advancements in Diagnostic Technologies
Improved diagnostic tools and screening methods—such as specific IgE testing and component-resolved diagnostics—enable earlier and more accurate detection of seasonal allergic rhinitis. Early diagnosis supports timely intervention and better patient outcomes.
-
Increased Healthcare Awareness and Access
Greater awareness about allergic conditions among patients and healthcare providers, coupled with better access to healthcare services, is driving higher diagnosis rates and treatment uptake.
-
Growing Focus on Personalized Medicine
Emerging trends in personalized and precision medicine are influencing treatment strategies. Tailored therapies based on patient-specific triggers and genetic profiles are gaining traction, enhancing treatment efficacy.
-
Aging Population
An aging global population is more susceptible to chronic respiratory and allergic conditions, driving demand for effective seasonal allergic rhinitis treatments and long-term management solutions.
-
Digital Health and Telemedicine
The adoption of telehealth services and digital symptom-tracking tools is improving patient engagement, monitoring, and treatment adherence, further boosting market growth.
DelveInsight’s report titled “Seasonal Allergic Rhinitis Market Insights, Epidemiology, and Market Forecast 2034” comprehensively analyzes Seasonal Allergic Rhinitis. The report includes a detailed examination of the historical and projected epidemiology data, including diagnosed prevalent cases of Seasonal Allergic Rhinitis segmented by age-specific, severity-specific, and allergen-specific cases. The Seasonal Allergic Rhinitis market report offers an in-depth understanding of the various aspects related to the patient population, including diagnosis, prescriptions patterns, physician perspectives, market access, treatment, and future market developments for the seven major markets, including the United States, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan from 2020 to 2034.In order to gauge the market’s overall potential and identify business opportunities, the report discusses current Seasonal Allergic Rhinitis treatment practices and algorithms as well as unmet medical needs.
Seasonal Allergic Rhinitis Treatment Market
Seasonal allergic rhinitis, also known as hay fever, is an allergic reaction that occurs during specific times of the year when the person comes in contact with a specific allergen. It is characterized by inflammation of the nasal passages, resulting in symptoms such as sneezing, itching, runny nose, and nasal congestion. Seasonal allergic rhinitis is typically triggered by the allergens present in the environment, such as trees, grass, weed pollen or dust mites, etc.
Diagnosing seasonal allergic rhinitis (hay fever) involves a comprehensive evaluation that includes obtaining a detailed medical history, physical examination, and allergy testing. The medical history focuses on understanding the nature, duration, and triggers of symptoms, while the physical examination assesses the nasal passages, throat, and eyes for signs of inflammation. Allergy testing, such as skin prick or blood tests, helps identify specific allergens that trigger symptoms.
Diagnosing seasonal allergic rhinitis presents multiple challenges, such as distinguishing its symptoms from those of other respiratory conditions requires thorough evaluation. Additionally, the availability and accessibility of allergy testing methods like skin prick tests or blood tests can vary, leading to possible delays and limitations in obtaining precise diagnostic information. In some cases, patients may have multiple allergies or sensitivities, making it more challenging to identify specific triggers.
The treatment of seasonal allergic rhinitis mainly involves antihistamines, decongestants, nasal corticosteroids, mast cell stabilizers, etc. For small populations, immunotherapy or biologics are also used. Treatment choice depends on symptom severity, individual preferences, and consultation with a healthcare professional.
The treatment of seasonal allergic rhinitis faces several challenges as medication responses vary among patients. Adherence to treatment regimens is also difficult, especially for long-term therapies such as nasal corticosteroids or immunotherapy, which require consistent use over an extended period. Additionally, the dynamic nature of allergen exposure, with seasonal fluctuations and variations in regional allergens, can also impact the effectiveness of treatment strategies.
Seasonal Allergic Rhinitis Epidemiology
The Seasonal Allergic Rhinitis epidemiology section provides insights into the historical and current Seasonal Allergic Rhinitis patient pool and forecasted trends for seven individual major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the report also provides the diagnosed patient pool, its trends, and assumptions undertaken.
The epidemiology section on the Seasonal Allergic Rhinitis market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
Key findings from the Seasonal Allergic Rhinitis Epidemiological Analysis
- Out of the 7MM, EU4 and the UK accounted for 43% of the Seasonal Allergic Rhinitis Diagnosed Prevalent Cases in 2022.
- Age-specific diagnosed prevalent cases of Seasonal Allergic Rhinitis in the following age groups, i.e., 0–10 years, 10–17 years, 18–59 years, and 60+ years, respectively.
- In 2022, the highest diagnosed prevalent cases of Seasonal Allergic Rhinitis among EU4 and the UK were observed in Germany, whereas Spain reported the minimum number of cases.
- Allergen-specific sensitivity is further distributed into various allergen groups, which include grass pollen, mites, tree pollen, animal dander, fungal spores, and weed pollen, to understand the frequently detected allergens in the Seasonal Allergic Rhinitis patient pool.
Seasonal Allergic Rhinitis Market Recent Breakthroughs
- In December 2025, Longbio Pharma announced a study was to evaluate the efficacy and safety of LP-003 in combination with SoC (nasal corticosteroids and/or anti-histamine) in adult patients with Moderate to Severe Seasonal Allergic Rhinitis, whose symptoms were inadequately controlled despite the current recommended therapies (nasal corticosteroids and/or anti-histamine) in the previous 2 pollen seasons.
- In February 2025, Lupin received FDA approval for its generic version of Boehringer Ingelheim's Atrovent Nasal Spray, 0.06%. The ipratropium bromide nasal solution is approved for symptomatic relief of rhinorrhea due to the common cold or seasonal allergic rhinitis in adults and children aged five and older.
Seasonal Allergic Rhinitis Market Outlook
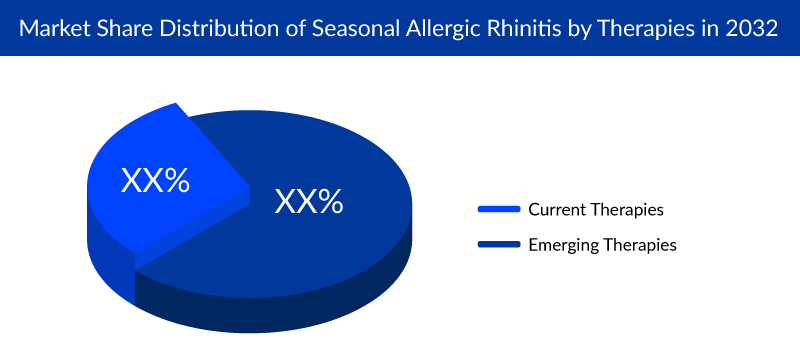
The current Seasonal Allergic Rhinitis Drugs Market has medications, including antihistamines, nasal sprays, and immunotherapies, whereas the emerging market shows novel treatments offering better symptom relief and targeted approaches. Drug therapy includes oral antihistamines to suppress runny noses through the inhibition of histamine action in the body, nasal steroids to suppress nasal inflammation, decongestants, and anticholinergic medications to provide temporary relief from nasal congestion, and leukotriene receptor antagonists to improve nasal congestion. In addition, mast cell stabilizers prevent the release of histamine and other inflammatory substances.
For certain cases, immunotherapy is also used, which involves subcutaneous or intravenous administrations of specific allergens. Additionally, the rising demand for over-the-counter allergy medications and the increasing use of generic options may contribute to market expansion. With ongoing research and innovation, the seasonal allergic rhinitis market will likely continue its positive trajectory, relieving millions of individuals affected by this condition.
"According to DelveInsight, the Seasonal Allergic Rhinitis Treatment Market in the 7MM is expected to change significantly during the study period 2020–2034 significantly"
Seasonal Allergic Rhinitis Marketed Drugs
-
RYALTRIS (olopatadine hydrochloride and mometasone furoate monohydrate): Glenmark Pharmaceuticals Inc.
RYALTRIS, a fixed-dose combination nasal spray containing an antihistamine and a steroid, was granted approval by the US FDA in January 2022 for treating symptoms related to seasonal allergic rhinitis in adults and pediatric patients aged 12 and above. Before this, in August 2021, Glenmark Pharmaceuticals obtained marketing approval for Ryaltris, its fixed-dose combination nasal spray, in 13 countries within the European Union and the United Kingdom.
-
XOLAIR (omalizumab): Novartis Pharmaceuticals
In December 2020, Novartis received approval for the additional XOLAIR (Omalizumab) indication for seasonal allergic rhinitis in Japan to treat severe Japanese cedar pollinosis. XOLAIR is a humanized monoclonal anti-IgE antibody administered subcutaneously and plays a vital role in developing allergic inflammation.
-
RAGWITEK/ RAGWIZAX: ALK-Abello
In April 2021, ALK-Abello obtained approval from the US FDA for RAGWITEK, a sublingual tablet containing short ragweed pollen allergen extract, for the treatment of allergic rhinitis caused by short ragweed pollen in individuals ranging from 5 to 65 years of age. Previously, in October 2020, it was approved under the brand name RAGWIZAX in European children.
Note: Detailed marketed therapies assessment will be provided in the final report.
Emerging Seasonal Allergic Rhinitis Drugs
The currently available Seasonal Allergic Rhinitis treatment aim to mitigate the complications associated with the condition. The Seasonal Allergic Rhinitis market dynamics are expected to change, primarily due to increased healthcare spending worldwide. Seasonal Allergic Rhinitis Market players such as Regeneron Pharmaceuticals, Revolo Biotherapeutics, Allergy Therapeutics, and Emergo Therapeutics are actively involved in developing Seasonal Allergic Rhinitis treatments.
-
REGN5713-5714-5715: Regeneron Pharmaceuticals
REGN5713-5714-5715 is currently developing as a treatment for seasonal allergic rhinitis caused by birch pollen allergy. This therapeutic candidate is a combination of three human monoclonal antibodies and works by specifically targeting the pollen allergen Bet v 1. It can be administered via IV or SC routes.
-
IRL201104: Revolo Biotherapeutics
IRL-201104 is under development for several indications, including seasonal allergic rhinitis. The drug candidate is a peptide derived from chaperonin 60.1 of Mycobacterium tuberculosis. It can be administered via IV or SC routes. The company has recently completed its trials in Phase II.
Seasonal Allergic Rhinitis Market Segmentation
DelveInsight’s ‘Seasonal Allergic Rhinitis Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Seasonal Allergic Rhinitis market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Seasonal Allergic Rhinitis Market Size by Countries
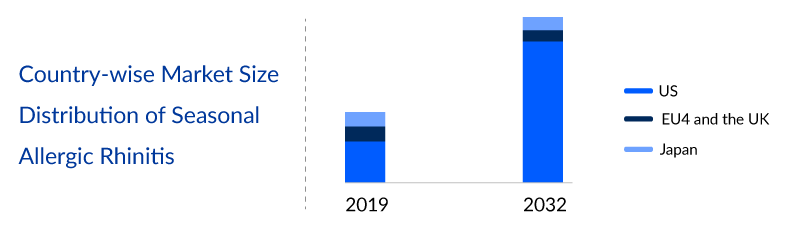
The total Seasonal Allergic Rhinitis market size is analyzed for individual countries (the United States Market, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan). The United States and EU4 and the UK accounted for a large portion of the 7MM market for Seasonal Allergic Rhinitis in 2022 due to the high prevalence of the condition, the high cost of treatments, and comparatively higher use of immunotherapy in the European countries. This dominance is predicted to continue with the potential early entry of new products.
Seasonal Allergic Rhinitis Market Size by Therapies
Various therapies, both over-the-counter medications and prescription drugs, are available within the seasonal allergic rhinitis market. These therapies encompass a range of options, such as antihistamines, nasal corticosteroids, decongestants, mast cell stabilizers, and immunotherapy. These treatments are commonly utilized to manage the symptoms associated with seasonal allergic rhinitis.
The seasonal allergic rhinitis market size by therapies faces several challenges as there is intense competition among pharmaceutical companies as they strive to develop and market innovative therapies to capture a larger market share. The overall market includes approved as well as generics. Antihistamines are a class of choice in seasonal allergic rhinitis, with the most preference for second-generation histamine antagonists. Moreover, decongestants can help relieve nasal congestion in people. Corticosteroids also share a significant patient share as the cases are usually given nasal steroids for long-term benefit. Other therapies include mast cell stabilizers, leukotriene receptor antagonists, etc. At present, several therapies have been approved in the 7MM, including XOLAIR by Novartis Pharmaceuticals, ODACTRA/ACARIZAX/MITICURE (SLIT-tablet) by ALK-Abello, GRASTEK/GRAZAX (Grass pollen allergy vaccine tablet) by ALK-Abello, ITULAZAX, RAGWITEK/RAGWIZAX, CEDARCURE by ALK-Abello, Stallergenes Greer’s ACTAIR (STG320) and ORALAIR, Glenmark Pharmaceuticals’ (RYALTRIS), BILAXTEN/BILANOA by FAES Farma/Taiho Pharmaceutical, etc.
The potential therapies in the emerging market are also robust and expected to bring about a positive shift in the treatment landscape, favoring the management of Seasonal Allergic Rhinitis.
Note: Detailed market segment assessment will be provided in the final report.
Seasonal Allergic Rhinitis Drugs Uptake
This section focuses on the sales uptake of potential Seasonal Allergic Rhinitis drugs that have recently launched or are anticipated to be launched in the Seasonal Allergic Rhinitis market between 2020 and 2034. It estimates the penetration Seasonal Allergic Rhinitis drug market for a given country, examining its impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Seasonal Allergic Rhinitis market. The emerging Seasonal Allergic Rhinitis therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry, and other market dynamics, as well as the unmet need they fulfill in the Seasonal Allergic Rhinitis market.
Seasonal Allergic Rhinitis Market Access and Reimbursement
DelveInsight’s ‘Seasonal Allergic Rhinitis Therapeutics Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Seasonal Allergic Rhinitis. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
Latest KOL Views on Seasonal Allergic Rhinitis
To keep up with current Seasonal Allergic Rhinitis market trends and fulfill gaps in secondary findings, we interview KOLs and SMEs’ working in the Seasonal Allergic Rhinitis domain. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or Seasonal Allergic Rhinitis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Seasonal Allergic Rhinitis unmet needs.
Seasonal Allergic Rhinitis KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as Clinique des Maladies respiratoires, Centre Hospitalier Universitaire, Montpellier in France; Division of Allergy and Immunology, University of California in the US.
“Sublingual immunotherapy has been empirically shown to have long-term therapeutic benefits. In terms of curative treatment, these therapies are promising as they are expected to continue to limit the SAR symptoms many years even after cessation of the treatment.”
“The most commonly prescribed classes of drugs for the treatment of seasonal allergic rhinitis are nasal corticosteroids and antihistamines.”
Seasonal Allergic Rhinitis Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Seasonal Allergic Rhinitis Market using various Competitive Intelligence tools, including SWOT analysis, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Seasonal Allergic Rhinitis Pipeline Development Activities
The Seasonal Allergic Rhinitis treatment market report provides insights into therapeutic candidates in Phase II and III stages. It also analyses Seasonal Allergic Rhinitis Companies involved in developing targeted therapeutics. The Seasonal Allergic Rhinitis treatment market report covers collaborations, acquisition and merger, licensing, patent details, and other information for emerging Seasonal Allergic Rhinitis therapies.
Seasonal Allergic Rhinitis Market Report Insights
- Patient-based Seasonal Allergic Rhinitis Market Forecasting
- Therapeutic Approaches
- Seasonal Allergic Rhinitis Pipeline Analysis
- Seasonal Allergic Rhinitis Market Size and Trends
- Seasonal Allergic Rhinitis Market Opportunities
- Impact of Upcoming Therapies
Seasonal Allergic Rhinitis Market Report Key Strengths
- 10-Year Seasonal Allergic Rhinitis Market Forecast
- 7MM Coverage
- Seasonal Allergic Rhinitis Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Seasonal Allergic Rhinitis Treatment Market
- Seasonal Allergic Rhinitis Drugs Uptake
Seasonal Allergic Rhinitis Market Report Assessment
- Current Seasonal Allergic Rhinitis Treatment Market Practices
- Seasonal Allergic Rhinitis Unmet Needs
- Seasonal Allergic Rhinitis Pipeline Product Profiles
- Seasonal Allergic Rhinitis Market Attractiveness
Key Questions Answered in the Seasonal Allergic Rhinitis Market
- What are the key findings of the market across 7MM, and which country will have the largest Seasonal Allergic Rhinitis market size during the forecast period (2024–2034)?
- At what CAGR is the Seasonal Allergic Rhinitis market, and is epidemiology expected to grow in the 7MM during the forecast period (2024–2034)?
- Are there any differences in the usage of immunotherapy across different regions of the 7MM?
- How would the unmet needs impact the Seasonal Allergic Rhinitis market dynamics and subsequently influence the analysis of related trends?
- What would be the forecasted patient pool of Seasonal Allergic Rhinitis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Which therapy class is likely to increase in patient share in the forecast period 2024–2034?
- What are the latest advancements in novel therapies, targets, mechanisms of action, and technologies being developed to address the limitations of existing therapies for Seasonal Allergic Rhinitis?
- How many companies are currently engaged in the development of therapies for the treatment of Seasonal Allergic Rhinitis?
Access Exclusive Data Now! Click here to Read More about the Related Articles:- New DelveInsight Blogs