Secondary Hyperparathyroidism Market
- The Secondary Hyperparathyroidism market is projected to witness consistent growth throughout the forecast period (2023–2032) due to the growing CKD population, increasing awareness and understanding of Secondary hyperparathyroidism leading to improved diagnosis and treatment rates.
- Analysts from DelveInsight have observed a significant surge in the prevalence of Secondary Hyperparathyroidism in recent times. Breakthrough discoveries and advancements in scientific knowledge are expected to drive market growth by introducing new therapeutic approaches.
- Several companies, such as Amgen Inc., OPKO Health, Inc. (OPK), and others, provide Secondary Hyperparathyroidism solutions. To propel the growth of the SHPT market in the coming years, several companies, such as Launch Therapeutics, Pathalys Pharma, Mitsubishi Tanabe Pharma, and others, are currently engaged in the development of drugs in early to mid-phase clinical trials. With the anticipated approval of these innovative therapies within the forecast period (2023–2032), the overall therapeutic market for Secondary Hyperparathyroidism is expected to experience a substantial increase, exhibiting a significant compound annual growth rate (CAGR).
Download the Sample PDF to Get More Insight @ Secondary Hyperparathyroidism Market
DelveInsight’s report titled “Secondary Hyperparathyroidism Market Insights, Epidemiology, and Market Forecast – 2032” comprehensively analyzes Secondary Hyperparathyroidism. The report includes a detailed examination of the historical and projected epidemiology data, including Prevalent Cases of Secondary Hyperparathyroidism (SHPT), Diagnosed and Treatable Cases of Secondary Hyperparathyroidism (SHPT). The Secondary Hyperparathyroidism market report offers an in-depth understanding of the various aspects related to the patient population, including diagnosis, prescription patterns, physician perspectives, market access, treatment, and future market developments for the seven major markets, including the United States, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan from 2019 to 2032.
In order to gauge the market’s overall potential and identify business opportunities, the report discusses current SHPT treatment practices and algorithms as well as unmet medical needs.
|
Study Period |
2019–2032 |
|
Forecast Period |
2023–2032 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Secondary Hyperparathyroidism (SHPT) Epidemiology |
|
|
Secondary Hyperparathyroidism (SHPT) Market |
|
|
Secondary Hyperparathyroidism Market Analysis |
|
|
Secondary Hyperparathyroidism Market Players |
Jiangsu Hengrui Medicine Co Ltd, Mitsubishi Tanabe Pharma Corp, OPKO Health Inc, Shaanxi Micot Technology Co Ltd, Amgen Inc, Cinkate Corp, Novadiol Inc, Scohia Pharma Inc, TaiRx Inc, Vidasym Inc., and others |
|
Class of Drugs |
|
Secondary Hyperparathyroidism Treatment Market
The diagnosis of Secondary Hyperparathyroidism is established through uncomplicated blood tests, which indicate low or standard blood calcium levels and elevated parathyroid hormone levels. Additionally, bone density scans (DXA) and X-rays may be employed to detect osteomalacia. These diagnostic procedures are conveniently conducted on an outpatient basis. Once the diagnosis of SHPT is confirmed, appropriate management strategies can be implemented to control symptoms and prevent further progression of the condition.
Secondary Hyperparathyroidism management includes vitamin D analogs, calcimimetics, and phosphate binders to restore calcium, phosphorus and the PTH levels within normal range. Early consultation with nephrologists is important to manage the outcomes. Surgery is the last option if patients do not respond to appropriate medical treatments. Despite advances in Secondary Hyperparathyroidism understanding, the optimal management of SHPT in non-dialysis CKD remains challenging. While there is increasing recognition of the need to identify and treat patients with SHPT earlier in the course of the disease, target levels of PTH are unclear, as are the levels of vitamin D required to achieve PTH reduction.
Secondary Hyperparathyroidism Overview
Secondary hyperparathyroidism is a medical condition in which the parathyroid glands secrete excessive amounts of parathyroid hormone (PTH) in response to hypocalcemia (low blood calcium levels), leading to the hyperplasia of these glands. The majority of patients with this condition have chronic renal failure. A common sign is discomfort in the bones and joints and limb abnormalities. Chronic kidney failure is the most common cause of secondary hyperparathyroidism.
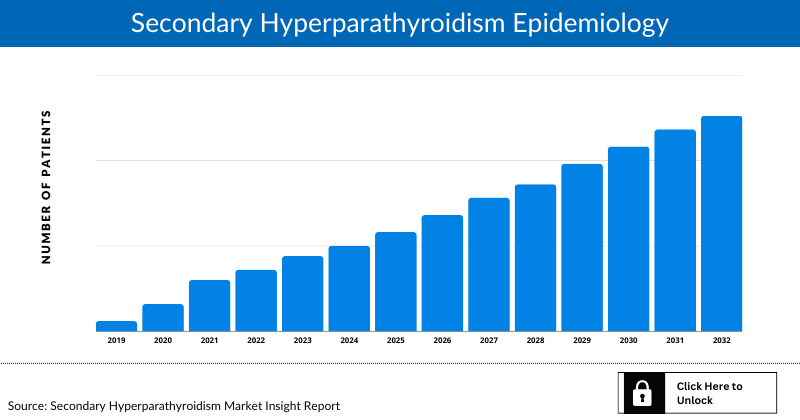
Secondary Hyperparathyroidism Epidemiology
The Secondary Hyperparathyroidism epidemiology section provides insights into the historical and current Secondary Hyperparathyroidism patient pool and forecasted trends for seven individual major countries. It helps recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the report also provides the diagnosed patient pool, its trends, and assumptions undertaken.
The epidemiology section on the Secondary Hyperparathyroidism (SHPT) market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
Key Findings
- According to the EPIRCE study conducted in Spain to measure the Secondary hyperparathyroidism prevalence and profile between diabetic and non-diabetic patients with Stage III–IV chronic kidney disease who attended internal medicine wards (MiPTH study), it was found that the prevalence of SHPT in CKD Stage III was 71.3% and 79.7% in Stage IV.
- As per studies carried out by Lorido et al. (2016), it was found that 60.4% of diabetic patients and 65% of non-diabetic patients suffered from SPTH among a subject population of 409 patients.
- Chronic Kidney Disease is the most common cause of SHPT. According to the recent US Renal Data System Report 2016, it was found that 56.3% of the prevalent dialysis patients were males, as were 59.7% of the kidney transplant recipients in the US.
- In a study conducted by Cozzolino et al. (2020), the estimated prevalence of SHPT, defined as iPTH > 300 pg/mL, in dialysis populations varies from approximately 30% to 50% across Europe, Asia, Oceania, and the Americas.
- According to the study conducted by Hedgeman et al. (2015), across Europe and Australia, the prevalence of SHPT within dialysis populations (PTH >300 pg/mL) ranged from 30% to 49%; prevalence within dialysis populations in America (US, Canada) was estimated at 54%. Within Asia, prevalence estimates for SHPT (iPTH >300 pg/mL) were only identified for India (28%) and Japan (11.5%).
Secondary Hyperparathyroidism Market Outlook
The therapeutic market for SHPT can be segmented across three main verticals: Vitamin D compounds (and its derivatives), Phosphate Binders, and Calcimimetics. Major player offering drug under this segment is Opko (RAYALDEE), Amgen (Etelcalcetide), Sanofi (Doxercalciferol), and others.
The pipeline is filled with exciting new therapies and expanded trials of already-approved drugs. Due to the emergence of calcimimetics and a better understanding of the strengths and limitations of native and active vitamin D compounds have improved the treatment options for patients with SHPT, the overall market sales are expected to show positive growth during the forecast period (2023–2032).
According to DelveInsight, the SHPT market in the 7MM is expected to significantly change during the study period 2019–2032.
Secondary Hyperparathyroidism Drug Chapters
Marketed Secondary Hyperparathyroidism Drugs
RAYALDEE: OPKO Health, Inc. (OPK)
RAYALDEE is a vitamin D3 analog indicated for treating secondary hyperparathyroidism in adults with Stage III or IV chronic kidney disease and serum total 25-hydroxyvitamin D levels less than 30 ng/mL. RAYALDEE is not indicated in patients with Stage V chronic kidney disease or end-stage renal disease on dialysis. RAYALDEE was approved by the US Food and Drug Administration (FDA) on June 17, 2016.
PARSABIV (etelcalcetide): Amgen
PARSABIV is a novel calcimimetic agent in clinical development for the treatment of sHPT in adult CKD patients on hemodialysis that is administered intravenously at the end of the hemodialysis session. PARSABIV binds to and activates the calcium-sensing receptor on the parathyroid gland, thereby decreasing PTH levels. In February 2017, the US Food and Drug Administration (FDA) approved PARSABIV (etelcalcetide) for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on hemodialysis.
Note: Detailed marketed therapies assessment will be provided in the final report...
Emerging Secondary Hyperparathyroidism Drugs
The Secondary Hyperparathyroidism market dynamics are expected to change, primarily due to increased healthcare spending worldwide. Secondary Hyperparathyroidism Market players such as Pathalys Pharma, Launch Therapeutics, and others are actively involved in developing Secondary Hyperparathyroidism treatments.
PLS240 (Upacicalcet): Launch Therapeutics/Pathalys Pharma
PLS240 is a novel calcimimetic that can potentially improve the treatment of secondary hyperparathyroidism (SHPT) in hemodialysis patients. By acting directly on parathyroid cell membrane calcium-sensing receptors, upacicalcet suppresses excessive parathyroid hormone (PTH) secretion, thereby lowering blood PTH levels. Launch Therapeutics/Pathalys Pharma is recruiting individuals for two Phase III trials (PATH-1 and PATH-2) in the US. It is already approved in Japan for the treatment of SHPT in patients on dialysis.
Note: Detailed emerging therapies assessment will be provided in the final report...
Secondary Hyperparathyroidism Market Segmentation
DelveInsight’s ‘Secondary Hyperparathyroidism (SHPT) Market Insights, Epidemiology, and Market Forecast – 2032’ report provides a detailed outlook of the current and future Secondary Hyperparathyroidism market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Secondary Hyperparathyroidism Market Size by Countries
The total Secondary Hyperparathyroidism market size is analyzed for individual countries (the United States Market, EU4 [Germany, France, Italy, and Spain] and the UK market, and Japan). The United States accounted for a larger portion of the 7MM market for SHPT in 2022 due to the high prevalence of the condition and the higher cost of treatments. This dominance is predicted to continue with the potential early entry of new products.
Secondary Hyperparathyroidism Market Size by Therapies
The Secondary Hyperparathyroidism market encompasses a range of therapies aimed at managing and treating this chronic condition characterized by an appropriate increase in parathyroid hormone (PTH) and in response to a stimulus, most commonly low serum calcium. The available treatment options for patients with Secondary Hyperparathyroidism (SHPT) have increased over the last two decades owing to the launch of calcimimetics and Vitamin D analogs. However, vigorous research activities are going on to expand the available patient base by including pediatric and CKD 5 patients for some of those drugs that have already been marketed.
Major companies, such as Opko (RAYALDEE), Amgen (Etelcalcetide), and Sanofi (Doxercalciferol), are some of the key players involved in expanding their already marketed product labels by focusing on pediatric and CKD V patient base. Estimates suggest that the launch of already existing marketed therapies with expanded labels and novel treatment options is expected to impact the treatment landscape during the forecast period positively.
Note: Detailed market segment assessment will be provided in the final report...
Secondary Hyperparathyroidism Drugs Uptake
This section focuses on the sales uptake of potential Secondary Hyperparathyroidism drugs that have recently launched or are anticipated to be launched in the SHPT market between 2019 and 2032. It estimates the market penetration of the Secondary Hyperparathyroidism drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Secondary Hyperparathyroidism market.
The emerging Secondary Hyperparathyroidism therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the SHPT market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Secondary Hyperparathyroidism...
Secondary Hyperparathyroidism Market Access and Reimbursement
DelveInsight’s ‘Secondary Hyperparathyroidism (SHPT) Market Insights, Epidemiology, and Market Forecast – 2032’ report provides a descriptive overview of the market access and reimbursement scenario of SHPT.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Secondary Hyperparathyroidism market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Secondary Hyperparathyroidism domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Secondary Hyperparathyroidism market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the SHPT unmet needs.
Secondary Hyperparathyroidism: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Department of Translational Medical Sciences, University of Campania ‘Luigi Vanvitelli,’ Italy; Endokrinologie in Charlottenburg, Berlin, Germany, and others.
“Secondary Hyperparathyroidism (SHPT) represents a significant complication in patients undergoing maintenance hemodialysis, who are also afflicted with a heightened risk of cardiovascular disease.”
“Patients affected by secondary hyperparathyroidism seem to have a more severe phenotype of primary aldosteronism (PA) and have a trend toward more cardiovascular comorbidities.”
Note: Detailed assessment of KOL Views will be provided in the full report of Secondary Hyperparathyroidism...
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Secondary Hyperparathyroidism Market using various Competitive Intelligence tools, including SWOT analysis, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Secondary Hyperparathyroidism Pipeline Development Activities
The Secondary Hyperparathyroidism report provides insights into Secondary Hyperparathyroidism Clinical Trials within Phase II and Phase III stages. It also analyses Secondary Hyperparathyroidism Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Secondary Hyperparathyroidism therapies.
Secondary Hyperparathyroidism Report Insights
- Secondary Hyperparathyroidism Patient Population
- Secondary Hyperparathyroidism Therapeutic Approaches
- Secondary Hyperparathyroidism Pipeline Analysis
- Secondary Hyperparathyroidism Market Size and Trends
- Secondary Hyperparathyroidism Market Opportunities
- Impact of Upcoming Secondary Hyperparathyroidism Therapies
Secondary Hyperparathyroidism Report Key Strengths
- 10 Years Forecast
- The 7MM Coverage
- Secondary Hyperparathyroidism Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Secondary Hyperparathyroidism Market
- Secondary Hyperparathyroidism Drugs Uptake
Secondary Hyperparathyroidism Report Assessment
- Secondary Hyperparathyroidism Current Treatment Practices
- Secondary Hyperparathyroidism Unmet Needs
- Secondary Hyperparathyroidism Pipeline Product Profiles
- Secondary Hyperparathyroidism Market Attractiveness
- Secondary Hyperparathyroidism Market Drivers
- Secondary Hyperparathyroidism Market Barriers
Key Questions Answered In The Secondary Hyperparathyroidism Market Report
- At what CAGR is the Secondary Hyperparathyroidism market, and is epidemiology expected to grow in the 7MM during the forecast period (2023–2032)?
- What are the key findings of the market across the 7MM, and what country will have the largest Secondary Hyperparathyroidism market size during the forecast period (2023–2032)?
- Are there any market barriers or challenges hindering the growth of the Secondary Hyperparathyroidism market (2023–2032)?
- What strategies are pharmaceutical companies adopting to capture a larger share of the Secondary Hyperparathyroidism (SHPT) market (2023–2032)?