Sepsis Market
- The Sepsis Market is projected to grow at a significant CAGR by 2034 in leading countries like the US, EU4, UK, and Japan.
- The leading Sepsis Companies such as La Jolla Pharmaceuticals, Par Pharmaceutical, Ono Pharmaceutical, Vivacelle Bio, Inotrem, Enlivex Therapeutics, Adrenomed, Shionogi, Asahi Kasei Pharma Corp., AM-Pharma, ABIONYX Pharma, Revimmune, Baxter Healthcare Corporation, BioMarck Pharmaceuticals, and others.
Request for Unlocking the Sample Page of the "Sepsis Treatment Market"
- The Sepsis Therapeutics Market in the 7MM is further expected to increase, attributed to the major drivers such as rising Sepsis Incidence population, technological advancements, increased funding for new drug development and Sepsis Clinical Trials, increasing awareness among common people, and growing research activities during the forecast period.
- The anticipated increase in Sepsis Incidence, coupled with the launch of promising emerging therapies like ALLOCETRA and enibarcimab, is set to drive significant growth in the Sepsis Diagnostic Market from 2024 to 2034, offering hope for improved treatment approaches.
- In April 2024, Enlivex announced the 28-topline data from the Phase II trial evaluating ALLOCETRA.
- In April 2024, AdrenoMed announced that the US FDA granted Fast Track designation to its lead product candidate, enibarcimab, a first-in-class non-neutralizing monoclonal antibody, for the Treatment of Septic Shock.
- In 2021, BioAegis Therapeutics Inc., a clinical-stage company, announced that it was awarded a contract from the Biomedical Advanced Research and Development Authority (BARDA) for the commercialization of gelsolin, a naturally occurring human protein that has exhibited precisely the properties that are required in Sepsis Drugs.
- The largest contributors to sepsis cases and sepsis-related mortality across all ages are diarrhoeal diseases and lower respiratory infections. However, non-communicable diseases are on the rise; one-third of sepsis cases and nearly half of all sepsis-related deaths are due to an underlying injury or chronic disease.
- Maternal disorders are the most common non-communicable disease complicated by sepsis. Among children, the most common causes of sepsis-related deaths are neonatal disorders, lower respiratory infections, and diarrhoeal diseases.
Request for unlocking the CAGR of the "Sepsis Drugs Market"
Key Factors Driving the Sepsis Market Growth
-
Rising Global Incidence of Sepsis
The increasing number of sepsis cases worldwide, driven by factors like aging populations, antimicrobial resistance, and hospital-acquired infections, significantly boosts the demand for effective diagnostic tools and therapeutic interventions. The high mortality and morbidity rates associated with sepsis further accelerate the need for advanced treatment options.
-
Growing Awareness and Early Diagnosis Initiatives
Government bodies, healthcare organizations, and NGOs are focusing on raising awareness about early identification and management of sepsis. The promotion of sepsis screening programs in hospitals and the introduction of sepsis awareness campaigns are contributing to early diagnosis and timely treatment, fueling market growth.
-
Technological Advancements in Diagnostics and Therapeutics
The introduction of rapid diagnostic technologies, point-of-care testing, and advanced molecular assays allows clinicians to detect sepsis pathogens faster and more accurately. In addition, the development of novel therapies, biologics, and immunomodulators is creating new growth opportunities for pharmaceutical players.
-
Regulatory Support and Incentives for Novel Therapies
Regulatory agencies like the FDA and EMA are offering fast-track designations, orphan drug status, and other incentives to encourage innovation in sepsis therapeutics. These supportive policies are accelerating product development and approvals, contributing to market expansion.
DelveInsight's “Sepsis Drugs Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Sepsis, historical and forecasted epidemiology as well as the Sepsis market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Sepsis Drugs Market Report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Sepsis Diagnostic Market Size from 2020 to 2034. The report also covers current Sepsis treatment market practices/algorithms and Sepsis unmet needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Sepsis Epidemiology |
Segmented by:
|
|
Sepsis Companies |
|
|
Sepsis Therapies |
|
|
Sepsis Therapeutics Market |
Segmented by:
|
|
Analysis |
|
Sepsis Treatment Market
Sepsis is a life-threatening organ dysfunction that results from the body’s response to infection. If not recognized early and managed promptly, it can lead to septic shock, multiple organ failure and death. Septic shock is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality. The initial evaluation of a patient with sepsis includes medical history, physical examination, and clinical symptoms, along with the detection of some biomarkers (such as C reactive protein and procalcitonin), which are crucial elements for early diagnosis of sepsis and the timely establishment of its appropriate clinical management. The Sepsis market outlook report provides an overview of Sepsis pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Sepsis Treatment
Sepsis treatment is examined under two categories with appropriate antimicrobial treatment and all-purpose supporting treatment. Antibiotics are mostly given empirically during the time until the determination of the active microorganism. The main target in septic shock treatment is regulating blood volume and providing sufficient tissue perfusion and tissues.Since all patients are different and the causes of sepsis are many, rapid administration of antibiotics and fluids in the first few hours would provide such means, and the addition of vasoactive drugs to the liquid treatment would benefit.
Various other therapies such as corticosteroids, dialysis, ventilation procedures, arterial lines, catheter, vasopressors, and others are recommended based on the need. Corticosteroids are another important agent shown to reduce death risk in sepsis treatment. By reducing tissue inflammation, triggering tissue repair and improving tissue perfusion, corticosteroids may prevent organ failure and reduce the intensity and number of organ dysfunction. Metabolic support is provided to regulate inflammation and acute phase response, and also to reduce morbidity and mortality rates. Organ-supporting treatments for respiratory failure and kidney failure treatments are used.
Sepsis Epidemiology
The Sepsis epidemiology chapter in the report provides historical as well as forecasted data in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2020 to 2034. The Sepsis epidemiology is segmented with detailed insights into Total Sepsis Incident cases, Sepsis Gender-specific Incident cases, Sepsis Severity-specific Incident Cases, and Sepsis Origin-specific Incident cases.
Key Findings from Sepsis Epidemiological Analyses and Forecast
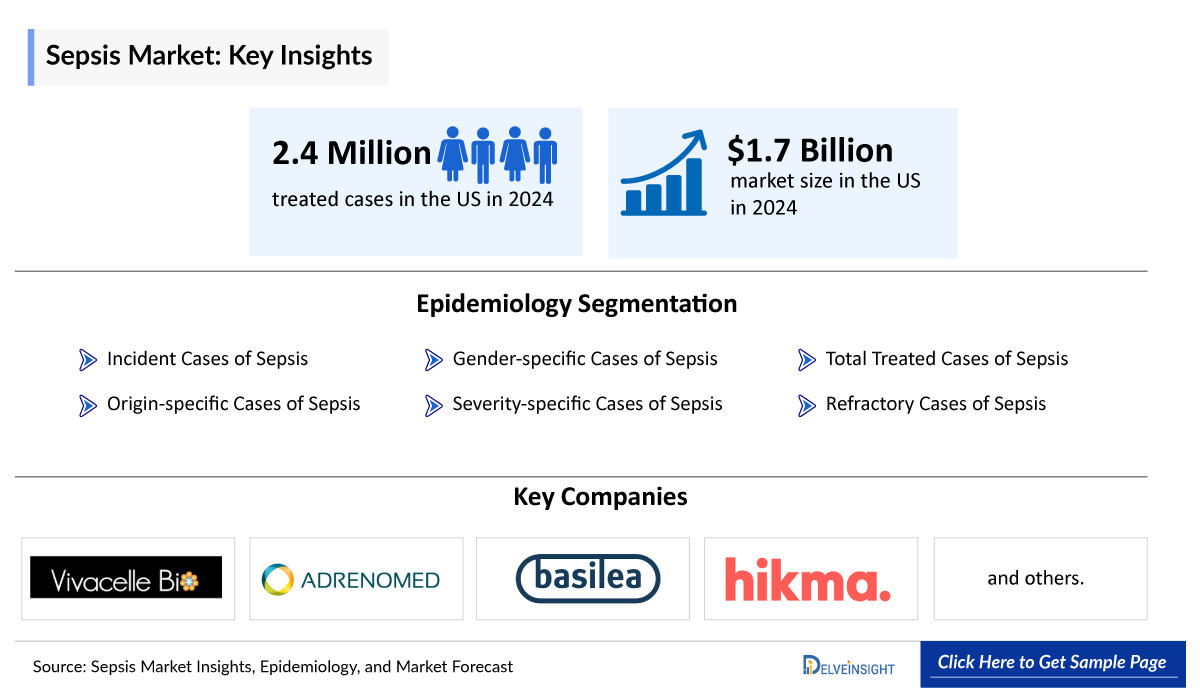
- Among 7MM, the United States accounted for the maximum share of the Sepsis population, with above 55% in 2023.
- In EU4 and the UK, Germany accounted for the highest number of incident cases in 2023.
- As per analysis, it is notable that in the 7MM, the majority of the patient pool was males as compared to females, whereas in the US female population dominated the Sepsis incident pool..
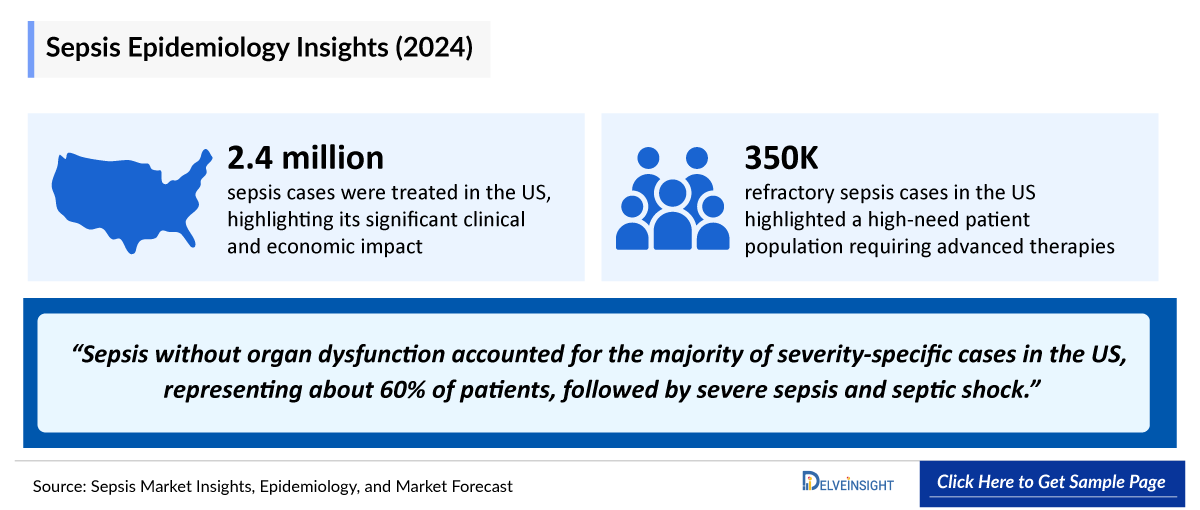
- According to Delveinsight, approximately 57% of all cases were sepsis without organ dysfunction, followed by septic shock (24%) and severe sepsis (18%) in the United States in 2023. These cases are expected to increase during the forecast period (2024-2034).
- As per the analysis, lung infection, UTI infection, gut infection, and skin infection are the leading four infection sites in patients with sepsis, accounting for approximately 43%, 30%, 13%, and 13%, respectively of all sites of infection.
Sepsis Epidemiology Segmentation
- Total Sepsis Incidence cases
- Sepsis Gender-specific Incidence Cases
- Sepsis Severity-specific Incidence Cases
- Origin-specific Sepsis Incidence Cases
Sepsis Market Recent Developments
- In April 2025, Vivacelle Bio announced that a study is being conducted to evaluate the safety and effectiveness of VBI-S in elevating the blood pressure of septic shock patients with absolute or relative hypovolemia. Sepsis is a serious condition resulting from the presence of harmful microorganisms in the blood or other tissues and the body's response to their presence, leading to the malfunctioning of various organs.
- In March 2025, Accelerate Diagnostics announced the submission of its Accelerate WAVE™ system and positive blood culture gram-negative test kit to the FDA for 510(k) clearance. The WAVE system offers rapid antimicrobial susceptibility testing (AST) from positive blood cultures and bacterial isolate colonies, providing accurate results in about 4.5 hours to enable same-shift targeted antimicrobial therapy for patients with serious infections.
Sepsis Drugs Analysis
The drug chapter segment of the Sepsis drugs market report encloses a detailed analysis of Sepsis marketed drugs and late-stage (Phase III and Phase II) sepsis pipeline drugs analysis. It also deep dives into the Sepsis clinical trials details, recent and expected market approvals, patent details, the latest Sepsis news, and recent deals and collaborations.
Sepsis Marketed Drugs
-
GIAPREZA (angiotensin II): La Jolla Pharmaceuticals/Innoviva Specialty Therapeutics
GIAPREZA (angiotensin II) (La Jolla Pharmaceuticals/Innoviva Specialty Therapeutics) injection is approved by the US FDA in December 2017 as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shocks. The drug is also approved by the European Commission in 2019 to treat refractory hypotension in adults with septic or other distributive shocks who remain hypotensive despite adequate volume restitution and application of catecholamines and other available vasopressor therapies. The drug mimics the body’s endogenous angiotensin II peptide, which is central to the renin–angiotensin–aldosterone system, which regulates blood pressure.
-
Onoact Injection (Landiolol hydrochloride): Ono Pharmaceuticals
Onoact is a short-acting β1 blocker that selectively blocks β1 receptors mainly found in the heart. It is for emergency treatment of intra-operative or post-operative tachyarrhythmia (atrial fibrillation, atrial flutter, sinus tachycardia) and treatment of tachyarrhythmia in left ventricular dysfunction (atrial fibrillation, atrial flutter). In June 2022, Onoact received approval for intravenous infusion 50mg/150mg, for the additional indication of tachyarrhythmia (atrial fibrillation, atrial flutter, and sinus tachycardia) associated with sepsis for a partial change in the approved items of the manufacturing and marketing approval in Japan.
|
Table 1: Comparison of key marketed drugs | ||||
|
Drug name |
Company |
Sepsis MOA |
RoA |
Approval |
|
GIAPREZA |
La Jolla Pharmaceuticals/Innoviva Specialty Therapeutics |
Vasoconstrictor |
Intravenous |
US: 2017 EU & UK: 2019 |
|
Onoact Injection |
Ono Pharmaceuticals |
β1 blocker |
Intravenous |
Japan: 2022 |
Sepsis Emerging Drugs
-
VBI-S: Vivacelle Bio
Vivacelle Bio’s lead candidate, VBI-S is made of small particles of specific lipid called micelles and liposomes to treat hypotension. VBI-S is an intravenously injectable fluid comprised of phospholipid nanoparticles that were specifically designed to shift the biophysical properties of the ’body’s fluid volume in hypovolemic shock, due to sepsis, from non-survival to survival. In July 2019, the company announced US FDA clearance to enroll patients into a Phase IIa Sepsis Clinical Trials of VBI-S to elevate blood pressure in subjects who have shock due to sepsis, later met 100% endpoints in Phase II. The therapy is currently under Phase III clinical evaluation to treat hypovolemia due to sepsis/septic shock.
-
Nangibotide: Inotrem
Inotrem’s Nangibotide is a synthetic peptide and first-in-class TREM-1 inhibitor. Nangibotide blocks the TREM-1-mediated immune dysregulations in sterile or infectious acute inflammatory syndromes. The drug restores a balanced inflammatory response and improves outcomes, particularly in those patients with high levels of TREM-1 pathway activation. The drug is currently under Phase II for the treatment of septic shock. This lead candidate has been granted EMA’s PRIority MEdicines (PRIME) scheme, and was also granted Fast-track status by the FDA for septic shock.
|
Table 2: Comparison of key emerging drugs | |||||
|
Drug name |
Company |
RoA |
MoA |
Phase |
Special Status |
|
VBI-S |
Vivacelle Bio |
Intravenous |
Volume expanding colloid |
III |
NA |
|
Nangibotide |
Inotrem SA |
Intravenous |
TREM-1 inhibitor |
II |
US: Fast-track Designation EU: PRIME |
Sepsis Market Outlook
Sepsis is a complex disease, which not only involves a wide array of causative agents but also results in different individual immune responses, causing various single or multiple organ dysfunction. Treatment for sepsis varies, depending on the site and the cause of the initial infection, the organs affected and the extent of any damage. Sepsis should be treated as a medical emergency and treated as quickly and efficiently as possible as soon as it has been identified.
Sepsis treatment is examined under two categories with appropriate antimicrobial treatment and all-purpose supporting treatment. Empressin is an argipressin-based vasopressor indicated for the treatment of catecholamine-refractory hypotension following septic shock in patients older than 18 years. Argipressin (also known as vasopressin, arginine-vasopressin, antidiuretic hormone) is a naturally produced human hormone that contracts the smooth muscles of the vascular system, thereby raising blood pressure and inducing water retention in the body.
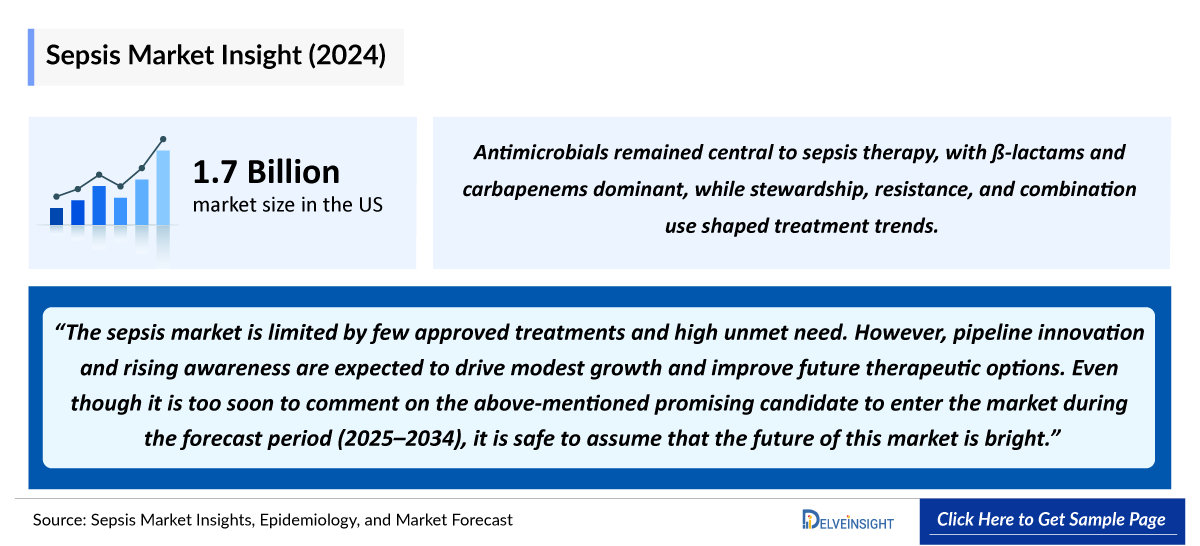
The Sepsis Therapeutics Market in the 7MM is anticipated to grow significantly between the forecasted period [2024-2034], driven by factors such as increasing Sepsis Incidence rates, technological advancements, enhanced funding for drug development, heightened public awareness, and expanding research endeavors. The expected launch of emerging therapies, combined with the rise in Sepsis cases, is poised to fuel market expansion throughout the forecast period. The promising pipeline for Sepsis presents a hopeful outlook for improved treatment modalities in the years ahead.
Sepsis Companies such as Vivacelle Bio’s, Inotrem, Enlivex Therapeutics, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Sepsis.
Key Findings from the Sepsis Market
- Among the 7MM, the United States accounted for the highest Sepsis Diagnostic Market Share in 2023.
- The launch of Sepsis Emerging Therapies, such as Allocetra by Enlivex Therapeutics, enibarcimab by Adrenomed, among others will significantly impact the Sepsis diagnostic market during the forecast period [2023-2034].
- The Sepsis Therapeutics Market in the 7MM is further expected to increase attributing to the major drivers such as rising incident population, technological advancements, increased funding for new drug development and Sepsis Clinical Trials, increasing awareness among common people, and increasing research activities during the forecast period.
- Medications with better safety and effectiveness, which provide an optimum cure, are the current unmet need of the sepsis therapeutics market.
Sepsis Drugs Uptake
This section focuses on the uptake rate of potential Sepsis drugs expected to be launched in the market during 2024–2034, which depends on the Sepsis Competitive Analysis Landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Sepsis companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Sepsis Pipeline Development Activities
The Sepsis pipeline segment provides insights into different therapeutic candidates in the Phase III and Phase II stages. It also analyzes Sepsis companies involved in developing targeted therapeutics. The Sepsis pipeline segment covers information on collaborations, acquisitions and mergers, licensing, and patent details for Sepsis emerging therapies.
Latest KOL Views on Sepsis
To keep up with the real-world scenario in current and Sepsis emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as Sepsis Alliance, Fulwood Hall Hospital etc., were contacted. Their opinion helps understand and validate current and emerging treatment patterns of Sepsis. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Sepsis Unmet Needs.
Sepsis KOL Views |
|
“Patients admitted to hospitals are being treated with intravenous antibiotics, intravenous fluids, oxygen, and blood work/testing to find the source of the infection. However, severe damage can take place in the surrounding tissue as well as organs if sepsis is diagnosed at a later stage.” |
|
“Several resources are available to treat injuries and conditions that cause mortality. Death due to sepsis can be reduced by educating and spreading awareness of the sudden onset and severity of the disease.” |
Sepsis Qualitative Analysis
We perform Qualitative and Sepsis therapeutics market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Sepsis Therapeutics Market Access and Reimbursement
The cost of newly approved medications is usually high, and because of it, patients can escape from proper treatment or opt for off-label and cheap medications. It affects the access of the newly launched medications in the market and reimbursement is a crucial factor. Often, the decision to reimburse comes down to the drug's price relative to the benefit it produces in treated patients. Market access and reimbursement options can differ depending on regulatory status, size of the target population, the setting of care, unmet need, magnitude of incremental benefit claims, and costs. La Jolla announced that the Centers for Medicare & Medicaid Services (CMS) had granted a New Technology Add-on Payment (NTAP) for GIAPREZA (angiotensin II) injection for intravenous infusion. The amount of the NTAP is equal to 50% of the amount by which the covered costs exceed the MS-DRG reimbursement, or 50% of the cost of the drug, whichever is less.
The Sepsis Treatment Drugs Market Report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Sepsis Treatment Drugs Market Report Scope
- The Sepsis Treatment Market Report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available Sepsis Therapies.
- Comprehensive insight has been provided into the Sepsis epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific Sepsis treatment market guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current Sepsis treatment market landscape.
- A detailed review of the Sepsis drugs market, historical and forecasted sepsis drugs market size, Sepsis treatment drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the Sepsis drugs market report, covering the 7MM drugs outreach.
- The Sepsis drugs market size report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Sepsis drugs market.
Sepsis Diagnostic Market Size Report Insights
- Patien-based Sepsis Market Forecasting
- Sepsis Therapeutics Market Approaches
- Sepsis Pipeline Analysis
- Sepsis Drugs Market Size
- Sepsis Drugs Market Trends
- Existing and future Sepsis Therapeutics Market Opportunity
Sepsis Treatment Drugs Market Report Key Strengths
- 11 Years Sepsis Market Forecast
- 7MM Coverage
- Sepsis Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging Sepsis Therapies
- Key Cross Competition
- Conjoint analysis
- Sepsis Drugs Uptake
- Key Sepsis Market Forecast Assumptions
Sepsis Treatment Drugs Market Size Report Assessment
- Current Sepsis Treatment Market Size Practices
- Sepsis Unmet Needs
- Sepsis Pipeline Drugs Profiles
- Sepsis Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Question Answered in the Sepsis Market Report
Sepsis Market Insights
- What is the growth rate of the 7MM Sepsis treatment market size?
- What was the Sepsis diagnostic market size, the Sepsis treatment market size by therapies, sepsis diagnostic market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the Sepsis treatment?
- How many companies are developing therapies for the Sepsis treatment?
- What are the recent novel therapies, targets, Sepsis Mechanisms of Action, and technologies developed to overcome the limitations of existing Sepsis Therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Sepsis Therapies?
Reasons to Buy the Sepsis Market Report
- The Sepsis drugs market size report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Sepsis drugs market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Sepsis therapeutics market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Sepsis therapeutics market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the Sepsis unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Access Exclusive Data Now! Click here to Read More about the Related Articles:-