Systemic Inflammatory Response Syndrome Market
DelveInsight's "Systemic Inflammatory Response Syndrome (SIRS) — Market Insights, Epidemiology, and Market Forecast — 2032" report delivers an in-depth understanding of the systemic Inflammatory Response Syndrome (SIRS), historical and forecasted epidemiology as well as the systemic inflammatory response syndrome (SIRS)market trends in the United States, EU4 (Germany, Spain, Italy, France) and the United Kingdom, and Japan.
The systemic inflammatory response syndrome (SIRS) market report provides current treatment practices, emerging drugs, systemic inflammatory response syndrome (SIRS) market share of the individual therapies, current and forecasted systemic inflammatory response syndrome (SIRS) market Size from 2019 to 2032 segmented by seven major markets. The Report also covers current systemic inflammatory response syndrome (SIRS) treatment practice/algorithm, and unmet medical needs to curate best of the opportunities and assesses the underlying potential of the market.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, Spain) and the United Kingdom)
- Japan
Study Period: 2019–2032
Systemic Inflammatory Response Syndrome (SIRS): Disease Understanding and Treatment Algorithm
The DelveInsight’s systemic inflammatory response syndrome (SIRS) market report gives a thorough understanding of systemic inflammatory response syndrome (SIRS) by including details such as disease definition, symptoms, causes, pathophysiology, diagnosis, and treatment.
Systemic inflammatory response syndrome (SIRS) is an exaggerated defense response of the body to a noxious stressor to localize and then eliminate the endogenous or exogenous source of the insult. It is a clinical syndrome characterized by systemic inflammation and widespread tissue injury and can be defined by several clinical variables including temperature >38°C or <35°C, heart rate >90 beats/min, respiratory rate >20 breaths/min or PCO2 < 32 mmHg, and WBC > 12000 cells/mm or <4000 cells/mm.
Treatment
There is no drug of choice for the treatment of SIRS. Medications target specific diagnosis, preexisting comorbidities and prophylaxis regimens for prevention of complications. Medical care includes the prompt initiation of pertinent laboratory testing and imaging studies after obtaining a history and performing a physical examination. Treatment should be focused on possible inciting causes of SIRS
Systemic Inflammatory Response Syndrome (SIRS) Epidemiology
The systemic inflammatory response syndrome (SIRS) epidemiology section provides insights about historical and current systemic inflammatory response syndrome (SIRS) patient pool and forecasted trends for individual seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the report also provides the diagnosed patient pool and their trends along with assumptions undertaken.
Key Findings
As per Brun-Buisson (2000), SIRS affected one-third of all in-hospital patients, and >50% of all ICU patients; in surgical ICU patients, SIRS occurred in >80% patients. About one-third of patients with SIRS had or evolved to sepsis. Sepsis occurred in approximately 25% of ICU patients, and bacteremic sepsis in 10%. In such patients, sepsis evolved to severe sepsis in >50% of cases, whereas evolution to severe sepsis in non-ICU patients was about 25%.
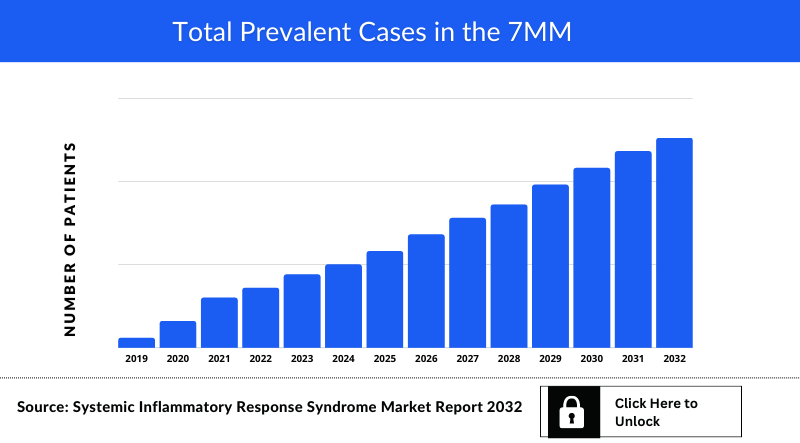
The disease epidemiology covered in the report provides historical as well as forecasted systemic inflammatory response syndrome (SIRS) epidemiology segmented as Total Incident cases of Systemic inflammatory response syndrome, Etiology-Specific Cases of Systemic inflammatory response syndrome (Infection, Trauma and others) and Treatable Patient Pool of Systemic inflammatory response syndrome in the 7MM covering the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2019 to 2032.
Country Wise- Systemic Inflammatory Response Syndrome (SIRS) Epidemiology
This section provides glimpse of the systemic inflammatory response syndrome (SIRS) epidemiology in the United States, EU4 (Germany, Spain, Italy, France) and United Kingdom, and Japan
Systemic Inflammatory Response Syndrome (SIRS) Recent Developments
- In April 2025, SeaStar Medical Holding Corporation (Nasdaq: ICU) announced that the U.S. FDA granted two Breakthrough Device Designations for its Selective Cytopheretic Device (SCD) therapy. The designations cover treating systemic inflammatory response in adult and pediatric cardiac surgery patients to help prevent post-operative complications.
Systemic Inflammatory Response Syndrome (SIRS) Drug Chapters
The drug chapter segment of the systemic inflammatory response syndrome (SIRS) report encloses the detailed analysis of systemic inflammatory response syndrome (SIRS) marketed drugs and late stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the systemic inflammatory response syndrome (SIRS) clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Note: Detailed Current therapies assessment will be provided in the full report of systemic inflammatory response syndrome (SIRS)
Systemic Inflammatory Response Syndrome (SIRS) Emerging Drugs
Auxora (CalciMedica), also known as CM-4620 Injectable Emulsion or CM-4620-IE, is a potent and selective small molecule inhibitor of Orai1-containing CRAC channels that is being developed for use in patients with acute inflammatory diseases. CRAC channels play a role in inflammation-induced injury of pulmonary endothelial cells, resulting in loss of alveolar-capillary barrier function and accumulation of fluid in the alveoli.
MultiStem (Athersys) is a patented and proprietary stem cell product candidate, which has the potential to treat many diseases and conditions, with a focus in neurological, inflammatory and immune disease areas, cardiovascular, and some pulmonary indications.
Note: Detailed emerging therapies assessment will be provided in the full report of systemic inflammatory response syndrome (SIRS)
Systemic Inflammatory Response Syndrome (SIRS) Market Outlook
Treatment is focused on possible inciting causes of SIRS (i.e. appropriate treatment of acute myocardial infarction will differ from the treatment of community acquired pneumonia or pancreatitis, etc.). It is recommended to avoid an antibiotic class to which a patient has recently been exposed. Care must be made not to use an antibiotic for which the patient is allergic. This may be a “second hit” and lead to worsening SIRS. Aztreonam is a reasonable alternative for gram negative bacteria in patients with significant penicillin allergies. Antiviral therapy has no role in SIRS. Empiric antifungal therapy (fluconazole or an echinocandin) may be considered in hemodynamically unstable patients who have already been treated with antibiotics, are neutropenic, receiving TPN or who have long term central venous access in place.
Key players such as Athersys, RevImmune along with few others are involved in developing therapies for systemic inflammatory response syndrome (SIRS).
According to DelveInsight, systemic inflammatory response syndrome (SIRS) market in 7MM is expected to witness a major change in the study period 2019-2032.
Analyst Commentary
-
The pipeline of systemic inflammatory response syndrome (SIRS) is very robust, many potential therapies are being investigated for the treatment of systemic Inflammatory Response Syndrome (SIRS), and it is safe to predict that the treatment space will experience a significant impact on the market during the forecast period.
- The expected introduction of emerging therapies with improved efficacy, more awareness initiatives programs, and further improvement in the diagnosis rate, are likely to boost the growth of the systemic inflammatory response syndrome (SIRS) market in the 7MM. Aside from that, the market size of systemic inflammatory response syndrome (SIRS) may flourish due to increased research and development, label-expansion of approved therapies into other epilepsy in this field.
- The market growth of systemic inflammatory response syndrome (SIRS) may offset by failures and/or discontinuation of the emerging therapies, unaffordable pricing, market access and reimbursement issues, and a scarcity of healthcare specialists.
Systemic Inflammatory Response Syndrome (SIRS) Drugs Uptake
This section focusses on the rate of uptake of the potential drugs recently launched in the systemic inflammatory response syndrome (SIRS) market or expected to get launched in the market during the study period 2019-2032. The analysis covers systemic inflammatory response syndrome (SIRS) market uptake by drugs; patient uptake by therapies; and sales of each drug.
This helps in understanding the drugs with the most rapid uptake, reasons behind the maximal use of new drugs, and allows the comparison of the drugs based on market share and size which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Note: Detailed emerging therapies assessment will be provided in full report of systemic inflammatory response syndrome (SIRS).
Systemic Inflammatory Response Syndrome (SIRS) Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase II and Phase III stage. It also analyses systemic inflammatory response syndrome (SIRS) key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing, patent details and other information for systemic inflammatory response syndrome (SIRS) emerging therapies.
Reimbursement Scenario in Systemic Inflammatory Response Syndrome (SIRS)
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In a report, we consider reimbursement to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
KOL- Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the systemic inflammatory response syndrome (SIRS) domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies treatment patterns or systemic inflammatory response syndrome (SIRS) market trends. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the market and the unmet needs.
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the systemic inflammatory response syndrome (SIRS) Market by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies etc. The inclusion of the analysis entirely depends upon the data availability.
|
Report Metrics |
Details |
|
Study Period |
2019 to 2032 |
|
Base Year |
2021 |
|
Forecast Period |
2022 to 2032 |
|
CAGR | |
|
Market Size | |
|
Key Companies | Cidera Therapeutics, ContraFect, Scynexis, Ono Pharmaceutical Co. Ltd, Pfizer, Takeda, Eli Lilly and Company, and others |
Scope of the Report
- The report covers the descriptive overview of systemic inflammatory response syndrome (SIRS), explaining its causes, signs and symptoms, pathophysiology, diagnosis, and currently available therapies
- Comprehensive insight has been provided into the systemic inflammatory response syndrome (SIRS) epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging therapies for systemic inflammatory response syndrome (SIRS) are provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of the systemic inflammatory response syndrome (SIRS) market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the global systemic inflammatory response syndrome (SIRS) market
Report Highlights
- In the coming years, the systemic inflammatory response syndrome (SIRS) market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The companies and academics are working to assess challenges and seek opportunities that could influence systemic inflammatory response syndrome (SIRS) R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing therapies for systemic inflammatory response syndrome (SIRS). The launch of emerging therapies will significantly impact the systemic inflammatory response syndrome (SIRS) market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for systemic inflammatory response syndrome (SIRS)
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends, and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Systemic Inflammatory Response Syndrome (SIRS) Report Insights
- Patient Population
- Therapeutic Approaches
- Systemic inflammatory response syndrome (SIRS) Pipeline Analysis
- Systemic inflammatory response syndrome (SIRS) Market Size and Trends
- Market Opportunities
- Impact of upcoming Therapies
Systemic Inflammatory Response Syndrome (SIRS) Report Key Strengths
- 11 Years Forecast
- 7MM Coverage
- Systemic inflammatory response syndrome (SIRS) Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Market
- Drugs Uptake
Systemic Inflammatory Response Syndrome (SIRS) Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
Key Questions
Market Insights:
- What was the systemic inflammatory response syndrome (SIRS) drug class share (%) distribution in 2019 and how it would look like in 2032?
- What would be the systemic inflammatory response syndrome (SIRS) total market size as well as market size by therapies across the 7MM during the forecast period (2019-2032)?
- What are the key findings of the market across 7MM and which country will have the largest systemic inflammatory response syndrome (SIRS) market size during the forecast period (2019-2032)?
- At what CAGR, the systemic inflammatory response syndrome (SIRS) market is expected to grow by 7MM during the forecast period (2019-2032)?
- What would be the systemic inflammatory response syndrome (SIRS) market outlook across the 7MM during the forecast period (2019-2032)?
- What would be the systemic inflammatory response syndrome (SIRS) market growth till 2032, and what will be the resultant market Size in the year 2032?
- How would the unmet needs affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights:
- What are the disease risk, burden, and regional/ethnic differences of systemic Inflammatory Response Syndrome (SIRS)?
- What are the key factors driving the epidemiology trend for seven major markets covering the United States, EU4 (Germany, Spain, France, Italy) and the UK, and Japan?
- What is the historical systemic inflammatory response syndrome (SIRS) patient pool in seven major markets covering the United States, EU4 (Germany, Spain, France, Italy) and the UK, and Japan?
- What would be the forecasted patient pool of systemic inflammatory response syndrome (SIRS) in seven major markets covering the United States, EU4 (Germany, Spain, France, Italy) and the UK, and Japan?
- Where will be the growth opportunities in the 7MM concerning the patient population about systemic Inflammatory Response Syndrome (SIRS)?
- Out of all 7MM countries, which country would have the highest incident population of systemic inflammatory response syndrome (SIRS) during the forecast period (2019-2032)?
- At what CAGR the patient population is expected to grow by 7MM during the forecast period (2019-2032)?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options for the systemic inflammatory response syndrome (SIRS) treatment in addition to the approved therapies?
- What are the current treatment guidelines for the treatment of systemic inflammatory response syndrome (SIRS) in the USA, Europe, and Japan?
- What are the systemic inflammatory response syndrome (SIRS) marketed drugs and their respective MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many companies are developing therapies for the treatment of systemic Inflammatory Response Syndrome (SIRS)?
- How many therapies are in-development by each company for systemic inflammatory response syndrome (SIRS) treatment?
- How many are emerging therapies in mid-stage, and late stage of development for systemic inflammatory response syndrome (SIRS) treatment?
- What are the key collaborations (Industry - Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the systemic inflammatory response syndrome (SIRS) therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for systemic inflammatory response syndrome (SIRS) and its status?
- What are the current challenges faced in drug development?
- What are the key designations that have been granted for the emerging therapies for systemic Inflammatory Response Syndrome (SIRS)?
- What are the global historical and forecasted markets of systemic Inflammatory Response Syndrome (SIRS)?
Reasons to buy
- The report will help in developing business strategies by understanding trends shaping and driving the systemic inflammatory response syndrome (SIRS) market
- Organize sales and marketing efforts by identifying the best opportunities for systemic inflammatory response syndrome (SIRS)in the US, Europe (Germany, Spain, Italy, France and the United Kingdom), and Japan
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for the systemic inflammatory response syndrome (SIRS) market
- To understand the future market competition in the systemic inflammatory response syndrome (SIRS) market