Tenosynovial Giant Cell Tumors Market
- The total Tenosynovial Giant Cell Tumor market size in the 7MM is approximately USD 300 million in 2023 and is projected to increase during the forecast period (2024–2034).
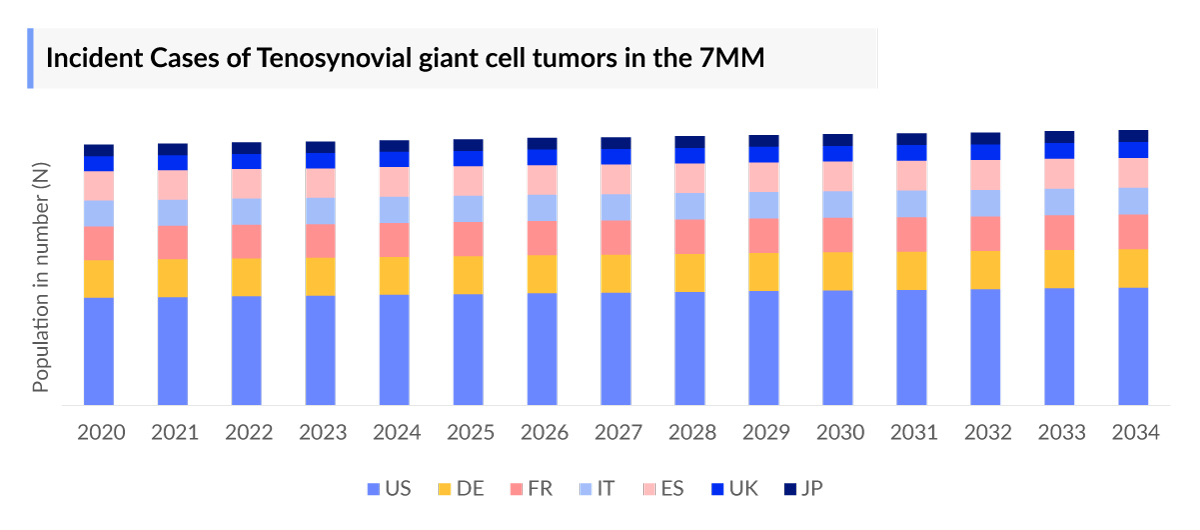
- Most Tenosynovial Giant Cell Tumor patients have a localized growth pattern, with approximately 12,610 incident cases in the United States.
- In 2023, the market size of Tenosynovial Giant Cell Tumor was the highest in the US among the 7MM, accounting for approximately USD 240 million, which is further expected to increase by 2034.
- Daiichi Sankyo’s TURALIO is the only FDA-approved Tenosynovial Giant Cell Tumors therapy. However, a significant number of patients still use an off-label treatment like imatinib due to TURALIO’s problematic safety and tolerability profile.
- In April 2024, Deciphera Pharmaceuticals revealed that it has signed a definitive merger agreement with ONO Pharmaceutical. Under this agreement, ONO will acquire all outstanding Deciphera common stock, followed by a merger of Deciphera with ONO's wholly-owned subsidiary.
- In June 2020, the European approval of TURALIO was rejected due to its potentially life-threatening hepatotoxic effects, leading to an unfavorable risk–benefit ratio in the treatment of the non-fatal condition of Tenosnovial Giant Cell Tumor.
- The expected launch of vimseltinib (Ono Pharmaceuticals) in 2025 is set to bring major changes in the treatment landscape of Tenosynovial Giant Cell Tumor, bringing in safer and more effective treatment options for Tenosynovial Giant Cell Tumor patients.
- TURALIO is covered by Medicare plans, typically listed on Tier 5 of their formulary, where patients must pay 25–33% of the retail cost for drugs.
- Other emerging therapies include SynOx Therapeutics’ Emactuzumab, Abbisko Therapeutics’ pimicotinib, AmMax Bio’s AMB-05X, and Elixiron Immunotherapeutics’ Enrupatinib.
Key Factors Driving Tenosynovial Giant Cell Tumor (TGCT) Market
Rising Tenosynovial Giant Cell Tumor Incidence
Most Tenosynovial Giant Cell Tumor patients exhibit a localized growth pattern, with 12.6K incident cases reported in the United States.
TURALIO’s Exclusive FDA Approval
Daiichi Sankyo’s TURALIO (Daiichi Sankyo) remains the only FDA-approved therapy for TGCT. However, its safety and tolerability challenges, including hepatotoxicity risks, have limited its widespread adoption, leading many patients to rely on off-label treatments such as imatinib. In 2023, the US TGCT market size was approximately USD 240 million, the highest among the 7MM, and is expected to expand considerably by 2034.
Regulatory and Strategic Developments
In June 2020, the European Medicines Agency (EMA) rejected TURALIO’s approval due to its unfavorable risk–benefit profile, particularly given the non-fatal nature of TGCT and its potential hepatotoxic effects. More recently, in April 2024, Deciphera Pharmaceuticals entered into a definitive merger agreement with ONO Pharmaceutical, under which ONO will acquire all outstanding shares of Deciphera, strengthening its position in oncology and rare disease markets.
Anticipated Launch of TGCT Emerging Therapies
The upcoming launch of Ono Pharmaceuticals’ vimseltinib in 2025 is expected to significantly transform the TGCT treatment landscape, offering patients a potentially safer and more effective option compared to TURALIO. Additionally, other promising pipeline candidates in development include SynOx Therapeutics’ Emactuzumab, Abbisko Therapeutics’ pimicotinib, AmMax Bio’s AMB-05X, and Elixiron Immunotherapeutics’ Enrupatinib, which collectively aim to address current unmet needs in safety, efficacy, and patient adherence.
DelveInsight’s “Tenosynovial Giant Cell Tumor Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of the Tenosynovial Giant Cell Tumor, historical and forecasted epidemiology as well as the Tenosynovial Giant Cell Tumor therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Tenosynovial Giant Cell Tumor market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Tenosynovial Giant Cell Tumor market size from 2020 to 2034. The report also covers current Tenosynovial Giant Cell Tumor treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Tenosynovial Giant Cell Tumor Market |
|
|
Tenosynovial Giant Cell Tumors Market Size | |
|
Tenosynovial Giant Cell Tumor Companies |
Ono Pharmaceutical Co. Ltd., SynOx Therapeutics, Celleron Therapeutics, Abbisko Therapeutics Co, Ltd, Merck, AmMax Bio, Inc., Elixiron Immunotherapeutics, Inc., and others. |
|
Tenosynovial Giant Cell Tumor Epidemiology Segmentation |
|
Tenosynovial Giant Cell Tumor Treatment Market
Tenosynovial Giant Cell Tumor is an abnormal growth of tissue derived from the synovium that causes activation of immune cells, specifically macrophages, leading to mass formation. These tumors are often classified by their growth pattern (localized or diffused) and location (intra-or extra-articular).
Recurrences are quite common in Tenosynovial Giant Cell Tumors, a major concern for decisions regarding treatment choice.
Tenosynovial Giant Cell Tumor Diagnosis
The diagnosis of tenosynovial giant cell tumor is based on identifying characteristic symptoms, a detailed patient history, a thorough clinical evaluation, and a variety of specialized tests. The initial symptoms of these tumors are often vague and may go unrecognized. Consequently, there is usually a significant delay from the onset of symptoms until a diagnosis is made. Imaging techniques like X-ray and MRI are frequently used to confirm the diagnosis of TGCT, but biopsy is also used in certain cases.
The current approach to diagnosing these patients is quite similar across the 7MM, where the journey begins with a referral to an ortho-oncologist by a primary care provider upon its diagnosis. Upon diagnosis, the patients are evaluated based on their tumor-growth pattern, and assessments are done to know if they are amenable to surgery.
Recurrences of localized tenosynovial giant cell tumors are observed more commonly in the EU4 countries and the UK than in countries of the 7MM, like the US and Japan.
Further details related to country-based variations are provided in the report...
Tenosynovial Giant Cell Tumor Treatment
Based on real-world data analysis, surgery is the most preferred treatment for tenosynovial giant cell tumors. Although surgery is quite effective for the management of tenosynovial giant cell tumors, recurrences after surgery are quite common. Despite surgeries being comparatively cheap compared to other immunotherapeutic options, there are significant other costs associated with very high surgeries. This ends up making the total cost of surgery quite expensive.
TURALIO is approved only in the US, while its approval in the EU got rejected. Daiichi Sankyo is also conducting trials to obtain its approval in Japan. TURALIO’s Marketing Authorization Application (MAA) was rejected by the European Commission (EC) because of its unfavorable risk–benefit ratio. TURALIO had potentially fatal hepatotoxic effects, although used to treat a non-fatal condition. Even in the US, its use is heavily monitored through the Risk Evaluation and Mitigation Strategy (REMS) Program, and its availability is restricted to certain specific centers.
Over the next few years, the US Tenosynovial Giant Cell Tumors Market is expected to change substantially and experience growth, as it will be dominated by upcoming products, vimseltinib, emactuzumab, pimicotinib, and AMB-05X. The safety and tolerability concerns of TURALIO are hindering its adoption in the treatment setting, with many patients preferring the usage of off-label TKIs like imatinib and mAbs like infliximab. Vimseltinib, emactuzumab, pimicotinib, and AMB-05X, with their significantly better safety profiles and improved efficacy, are expected to be readily adopted in the treatment setting.
Tenosynovial Giant Cell Tumor Epidemiology
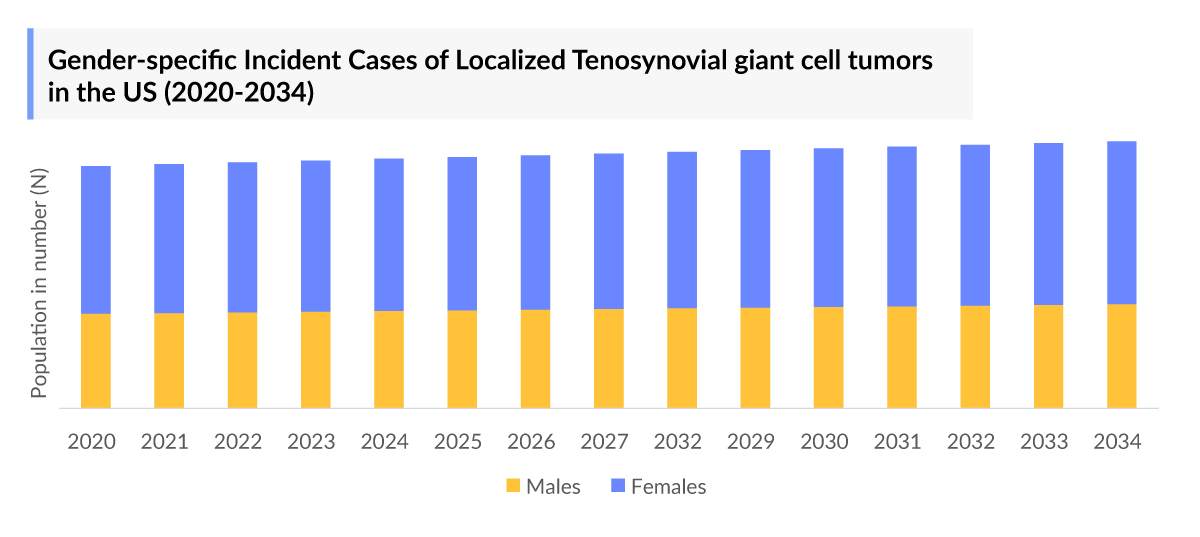
As the market is derived using a patient-based model, the Tenosynovial Giant Cell Tumor epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by incident cases of Tenosynovial Giant Cell Tumor, growth-pattern specific incident cases of Tenosynovial Giant Cell Tumor, gender-specific incident cases of localized Tenosynovial Giant Cell Tumor, gender-specific incident cases of diffuse Tenosynovial Giant Cell Tumor, tumor localization of localized Tenosynovial Giant Cell Tumor, tumor localization of diffuse Tenosynovial Giant Cell Tumor, and the total treated cases of Tenosynovial Giant Cell Tumor in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034. The total prevalent cases of Tenosynovial Giant Cell Tumor in the 7MM are projected to increase during the forecasted period.
- The total Tenosynovial Giant Cell Tumors prevalence in the United States was around 2,50,000 in 2023.
- The United States contributed to the largest incident population of Tenosynovial Giant Cell Tumor, acquiring ~43% of the 7MM in 2023. Whereas EU4, the UK, and Japan accounted for around 43% and 14% of the total incident population share, respectively, in 2023.
- Among the EU4 countries, Germany accounted for the largest number of Tenosynovial Giant Cell Tumor incident cases, followed by France, whereas Spain accounted for the lowest number of incident cases in 2023.
- According to DelveInsight estimates, there were around 1,98,000 prevalent cases of localized and 51,000 prevalent cases of diffuse Tenosynovial Giant Cell Tumor in the United States in 2023. The Tenosynovial Giant Cell Tumors prevalence is projected to increase during the forecasted period.
- In EU4 and the UK, approximately 36% of the localized Tenosynovial Giant Cell Tumors incidence is expected to recur, whereas only 50% of the incident cases of diffuse Tenosynovial Giant Cell Tumors are expected to recur.
Further details related to epidemiology will be provided in the report...
Recent Developments in the Tenosynovial Giant Cell Tumor Market:
- In April 2025, SynOx Therapeutics announced that the FDA granted Fast Track Designation to emactuzumab for the treatment of Tenosynovial Giant Cell Tumours (TGCT) in patients who are not amenable to or would not benefit from surgery. Emactuzumab, a CSF-1 receptor inhibiting monoclonal antibody, is being evaluated in the TANGENT Phase 3 trial.
- In March 2025, the FDA approved Stoboclo (CT-P41, denosumab-bmwo) and Osenvelt (CT-P41, denosumab-bmwo), biosimilars referencing Prolia (denosumab) and Xgeva (denosumab), for protecting bone health in cancer patients undergoing treatment. Xgeva is also approved for treating giant cell tumors of the bone that cannot be surgically removed and hypercalcemia of malignancy that worsens after bisphosphate treatment, as stated by the National Cancer Institute.
- In February 2025, Ono Pharmaceutical announced that the FDA approved ROMVIMZA™ (vimseltinib), a kinase inhibitor, for adult patients with symptomatic tenosynovial giant cell tumor (TGCT) where surgery may worsen functional limitations or cause severe morbidity. ROMVIMZA, developed by Deciphera Pharmaceuticals, received Fast Track designation and Priority Review from the FDA.
Tenosynovial Giant Cell Tumor Drug Chapters
The drug chapter segment of the Tenosynovial Giant Cell Tumor market report encloses a detailed analysis of Tenosynovial Giant Cell Tumor marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the Tenosynovial Giant Cell Tumor clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, each drug's advantages and disadvantages, and the latest news and press releases.
Tenosynovial Giant Cell Tumor Marketed Drugs
TURALIO (pexidartinib): Daiichi Sankyo
TURALIO (pexidartinib) is an orally administered small molecule tyrosine kinase inhibitor that targets colony-stimulating factor 1 receptor (CSF1R), KIT proto-oncogene receptor tyrosine kinase (KIT), and FMS-like tyrosine kinase 3 (FLT3) harboring an internal tandem duplication (ITD) mutation. The US FDA approves it for treating adult patients with symptomatic tenosynovial giant cell tumors associated with severe morbidity or functional limitations which are not amenable to improvement with surgery.
TURALIO received approval for the treatment of tenosynovial giant cell tumor patients in August 2019 in the United States. A new dosing regimen of TURALIO was introduced in February 2023, where the 200 mg capsule was discontinued and replaced by a 125 mg capsule. The new recommended dose, which the FDA approved in October 2022, is 125 mg capsules taken twice daily with a low-fat meal, instead of the former 200 mg capsules taken twice daily on an empty stomach. This was done to reduce the toxic effects of the drug.
TURALIO is expected to face heavy competition from therapies such as Ono Pharmaceuticals’ vimseltinib, SynOx Therapeutics’ emactuzumab, Abbisko Therapeutics’ pimicotinib, and AmMax Bio’s AMB-05X, which have demonstrated significantly better safety and efficacy profiles than TURALIO in clinical trials.
Note: Detailed current therapies assessment will be provided in the full report of Tenosynovial Giant Cell Tumor.
Tenosynovial Giant Cell Tumor Emerging Drugs
Vimseltinib (DCC-3014): Ono Pharmaceuticals
Vimseltinib (DCC-3014), which is being developed by Ono Pharmaceuticals, is an orally administered, potent, and highly-selective switch-control kinase inhibitor of the colony-stimulating factor 1 receptor (CSF1R) with potential antineoplastic, macrophage checkpoint-inhibitory and immunomodulating activities. The drug could be the best-in-class treatment for tenosynovial giant cell tumor patients. The Phase I/II study demonstrated promising preliminary results, encouraging antitumor activity with clinical benefits and manageable adverse events. These results supported its continued evaluation in a Phase III MOTION study, which, if successful, will lead to the FDA approval of the drug before 2026. Key opinion Leaders (KOLs) are convinced with the tolerability and antitumor activity of vimseltinib in clinical trials and have shown a preference toward vimseltinib over imatinib and pexidartinib, for the treatment of tenosynovial giant cell tumors.
Note: Detailed emerging therapies assessment will be provided in the final report...
Tenosynovial Giant Cell Tumor Drug Class Insights
The existing Tenosynovial Giant Cell Tumor treatment is mainly dominated by classes such as CSF1R inhibitors and TNF alpha inhibitors.
The approval of TURALIO for tenosynovial giant cell tumors treatment established the efficacy of colony-stimulating factor 1 receptor (CSF1R) targeting the treatment of TGCT. Although TURALIO established the efficacy of CSF1R inhibition, it also had concerns regarding its potentially fatal hepatotoxic effects. Hence emerging CSF1R inhibitors are currently focusing more on improving their safety profiles.
Although large-scale clinical trials have not established the effectiveness of TNF alpha inhibition, they are still used as off-label in certain cases to manage tenosynovial giant cell tumors.
CSF1R inhibitors dominate the upcoming treatment landscape.
Tenosynovial Giant Cell Tumor Market Outlook
Tenosynovial Giant Cell Tumor treatment in the US has entered a new era, with the dynamics expected to change. Surgery is the primary Tenosynovial Giant Cell Tumors therapeutic option, but it is frequently accompanied by recurrences and hampering the patient's functional status. When the tumor is removed as a whole, the likelihood of the recurrence of the tumor is reduced; however, complete removal of the tumor sometimes requires the mandatory undesirable removal of the functional structures of the patient. But if the tumor is removed partially to maintain a patient's functional status, recurrences are much more likely to appear.
TURALIO provided a new treatment option for patients with a significant need for systemic treatment. Its approval was set to solve a huge unmet need for treatment in patients with severe morbidity who were not amenable to surgery. Although it faced significant challenges regarding its adoption in the market due to its concerning safety and tolerability profile, causing fatal hepatotoxic effects in a non-fatal conditions. Due to the safety concerns of turalio, off-label treatments like imatinib are used frequently to treat tenosynovial giant cell tumors, although their efficacy is very limiting.
Currently, TURALIO is also being explored in the perioperative setting, where it is used in the neoadjuvant setting for tumor shrinkage, to help in appropriate resection, and in the adjuvant setting to prevent recurrences post-resection.
Patients and physicians have long cited a desire for effective therapy for tenosynovial giant cell tumors without sacrificing its safety and tolerability. The expected launch of upcoming therapies and a high unmet need for a therapy with a better safety profile than TURALIO will eventually facilitate the development of effective treatment options. However, the diagnosis of tenosynovial giant cell tumor is often associated with diagnostic delays and misdiagnosis due to its slow-growing nature, not well-differentiated symptoms, and lack of awareness of the disease among patients and providers. These factors often become a hindrance for both physicians and patients when adopting newer therapies.
Key tenosynovial giant cell tumor companies such as Ono Pharmaceuticals (vimseltinib), SynOx Therapeutics (emactuzumab), and others are evaluating their lead candidates in different stages of clinical development respectively. They aim to investigate their products Tenosynovial Giant Cell Tumor treatment.
- The total Tenosynovial Giant Cell Tumor market size in the 7MM is approximately USD 300 million in 2023 and is projected to increase during the forecast period (2024–2034).
- The Tenosynovial Giant Cell Tumors market size in the 7MM will increase at a CAGR of 19.1% due to increasing awareness of the disease and the launch of emerging therapies.
- Among EU4 countries, Germany accounts for the maximum market size in 2023, while Spain occupies the bottom of the ladder 2023.
- In Japan, there are no approved Tenosynovial Giant Cell Tumors therapies, with current treatment being done primarily by surgery, but off-label systemic therapies are also used in certain cases.
Tenosynovial Giant Cell Tumor Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. For example, for vimseltinib, we expect the drug uptake to be medium with a probability-adjusted peak share of 20%; years to the peak is expected to be 8 years from the year of launch.
Further detailed analysis of emerging therapies drug uptake in the report…
Tenosynovial Giant Cell Tumor Pipeline Development Activities
The report provides insights into Tenosynovial Giant Cell Tumor clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Tenosynovial Giant Cell Tumor emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SME’s opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Tenosynovial Giant Cell Tumor evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility, include Medical/scientific writers, Medical Oncologists, Professors, Medical Oncologists of the MD Anderson Cancer Center, Orthopaedic Oncologists of the Uniklinik Essen, and Others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers such as MD Anderson Cancer Center, Nuffield Orthopaedic Centre, Fondazione IRCCS Istituto Nazionale Tumori, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Tenosynovial Giant Cell Tumor market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Tenosynovial Giant Cell Tumors treatment market.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, administration frequency, administration route, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in Tenosynovial Giant Cell Tumor clinical trials, one of the most important primary outcome measures is the objective response rate of the therapy on TGCT patients.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement of rare disease therapies can be limited due to lack of supporting policies and funding, challenges of high prices, lack of specific approaches to evaluating rare disease drugs given limited evidence, and payers’ concerns about budget impact. The high cost of rare disease drugs usually has a limited impact on the budget due to the small number of eligible patients being prescribed the drug. The US FDA has approved several rare disease therapies in recent years. From a patient perspective, health insurance and payer coverage guidelines surrounding rare disease treatments restrict broad access to these treatments, leaving only a small number of patients who can bypass insurance and pay for products independently.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs etc.
Scope of the Tenosynovial Giant Cell Tumor Market Forecast Report
- The Tenosynovial Giant Cell Tumors market report covers a segment of key events, an executive summary, descriptive overview of Tenosynovial Giant Cell Tumor, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current treatment landscape.
- A detailed review of the Tenosynovial Giant Cell Tumor market, historic and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Tenosynovial Giant Cell Tumor market.
Tenosynovial Giant Cell Tumor Report Insights
- Patient Population
- Therapeutic Approaches
- Tenosynovial Giant Cell Tumor Pipeline Analysis
- Tenosynovial Giant Cell Tumor Market Size and Trends
- Existing and future Market Opportunity
Tenosynovial Giant Cell Tumor Report Key Strengths
- Ten Years Forecast
- 7MM Coverage
- Tenosynovial Giant Cell Tumor Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Market Forecast Assumptions
Tenosynovial Giant Cell Tumor Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered In The Tenosynovial Giant Cell Tumor Market Report:
Tenosynovial Giant Cell Tumor Market Insights
- What was the Tenosynovial Giant Cell Tumor total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors for this growth?
- How will CSF1R inhibitors as a class affect the treatment paradigm of Tenosynovial Giant Cell Tumor?
- What kind of uptake are CSF1R inhibitors set to witness in TGCT patients in the coming 10 years?
- What will be the impact of emerging therapies on off-label treatment usage?
- How will vimseltinib compete with pexidartinib and emactuzumab?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Tenosynovial Giant Cell Tumor Epidemiology Insights
- What are the disease risk, burdens, and unmet needs of Tenosynovial Giant Cell Tumor? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Tenosynovial Giant Cell Tumor?
- What is the historical and forecasted Tenosynovial Giant Cell Tumor patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- Why do so many patients recur after surgery?
- What factors are affecting the increase in Tenosynovial Giant Cell Tumor incident cases?
Current Tenosynovial Giant Cell Tumor Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Tenosynovial Giant Cell Tumor?
- How many companies are developing therapies for the treatment of Tenosynovial Giant Cell Tumor?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of Tenosynovial Giant Cell Tumor?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What have key designations been granted for the emerging therapies for Tenosynovial Giant Cell Tumor?
- What will be the impact of vimseltinib and emactuzumab on off-label imatinib usage?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted market of Tenosynovial Giant Cell Tumor?
Reasons to buy Tenosynovial Giant Cell Tumor Market Forecast Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Tenosynovial Giant Cell Tumor Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.
Related Reports:
-market.png&w=256&q=75)
.jpg)

-pipeline.png&w=256&q=75)



.png&w=3840&q=75)
