Thymidine Kinase 2 Deficiency Market Summary
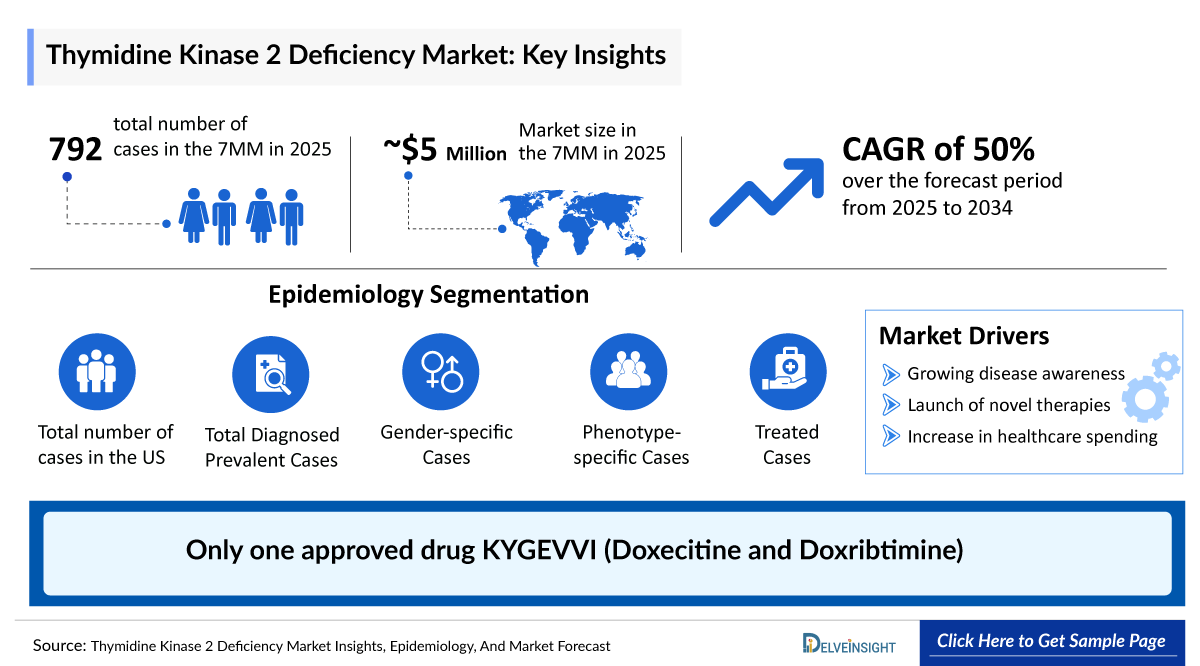
- The Thymidine Kinase 2 Deficiency Market in the 7MM is expected to grow from USD 5 million in 2025 to USD 210 million in 2034.
- The Thymidine Kinase 2 Deficiency Market is projected to grow at a CAGR of 50% by 2034 in leading countries like the US, EU4, UK, and Japan.
Thymidine Kinase 2 Deficiency Market and Epidemiology Analysis
- The Thymidine Kinase 2 Deficiency Market is expected to experience steady growth, with a strong compound annual growth rate (CAGR) forecasted from 2024 to 2034. This growth across the 7MM will be propelled by the introduction of innovative therapies such as MT1621 (Doxecitine and Doxribtimine) and others. Furthermore, the rising Thymidine Kinase 2 Deficiency prevalence, driven by increasing awareness and risk factors like advancements in diagnostic tools, the Thymidine Kinase 2 Deficiency market is poised for significant growth. Increasing focus on novel therapies and rehabilitation technologies further supports market expansion.
- According to DelveInsight, In 2023, the United States holds the largest Thymidine Kinase 2 Deficiency Drugs Market Share, accounting for approximately 60%, compared to the EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- TK2d was first described in 2001 in four children with severe muscle disease. All Thymidine Kinase 2 Deficiency Patients described have some degree of muscle weakness, but the severity, age of onset, and disease progression vary from person to person.
- Currently, there are no globally approved therapies that specifically target TK2d, leaving treatment primarily focused on symptom management through a multidisciplinary approach.
- TK2d is diagnosed through symptoms, patient history, clinical exam, and laboratory and genetic tests. Genetic testing for mutations in the TK2 gene confirms the diagnosis. Elevated creatine kinase levels and electromyography showing myopathic changes can support the diagnosis. Genetic counseling is recommended for affected individuals and their families.
- In 2023, the United States represented around 50% of the Thymidine Kinase 2 Deficiency Prevalence Cases in the 7MM, the highest among them. In 2023, the United States had the highest number of treated TK2d cases in the 7MM, with approximately 350 cases, followed by Germany with around 90 cases. These numbers are expected to rise during the forecast period.
- Thymidine Kinase 2 Deficiency Pipeline is not so robust but possesses one potential drug, i.e., MT1621 (Doxecitine and Doxribtimine).
Thymidine Kinase 2 Deficiency Market Size and Forecast
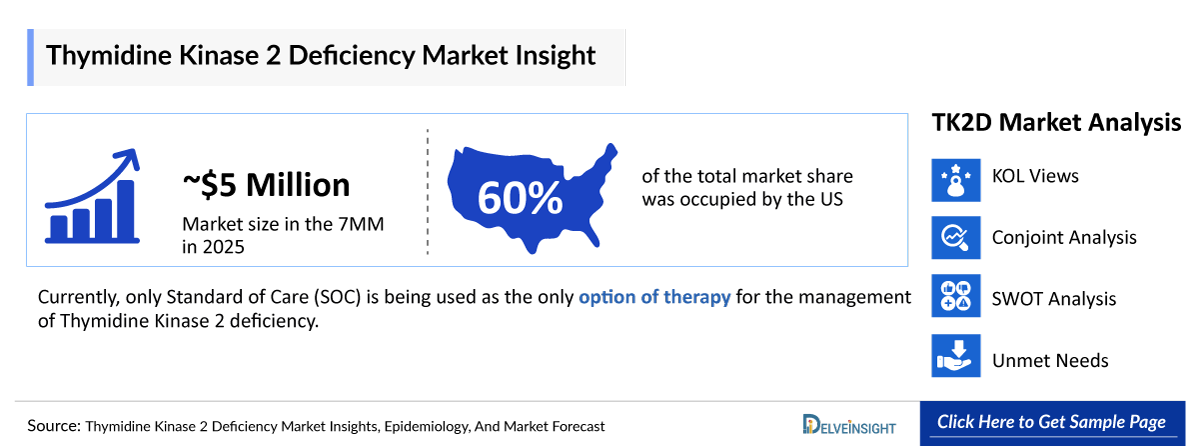
- 2025 Thymidine Kinase 2 Deficiency Market Size: ~USD 5 Million in 2025
- 2034 Projected Thymidine Kinase 2 Deficiency Market Size: ~USD 210 Million in 2034
- Growth Rate (2025-2034): 50% CAGR
- Largest Thymidine Kinase 2 Deficiency Market: United States
Key Factors Driving the Thymidine Kinase 2 Deficiency Market
Rising Thymidine Kinase 2 Deficiency Patient Pool
The prevalence of Thymidine Kinase 2 Deficiency (TK2d) is increasing across the 7MM, supported by improved awareness and advancements in diagnostic tools such as genetic testing. In 2023, the United States accounted for nearly 560 cases, representing around 50% of the total prevalence in the 7MM. With infantile-, childhood-, and late-onset subtypes all contributing to the burden, these numbers are projected to rise during the forecast period, expanding the treatable population.
Strong Growth Potential in the TK2d Therapeutic Market
The TK2d market in the 7MM is expected to grow at a remarkable 50% CAGR between 2025 and 2034, rising from USD 5 million in 2025 to USD 210 million by 2034. The United States dominates the market, holding nearly 60% share in 2023, making it the most significant commercial opportunity. Market growth will be fueled by improved diagnosis and the introduction of novel targeted therapies.
Thymidine Kinase 2 Deficiency Competitive Landscape
The current treatment landscape for TK2d relies mainly on supportive and symptomatic management through a multidisciplinary approach. However, the outlook is set to shift with the emergence of MT1621 (deoxycytidine + deoxythymidine), the leading candidate in late-stage development. MT1621 is designed to target the underlying mitochondrial dysfunction in TK2d and holds strong potential for rapid adoption once approved. Academic collaborations and ongoing research in rare mitochondrial myopathies and nucleoside-based therapies further enrich the competitive space, aiming to reduce the high unmet need in this rare disorder.
DelveInsight’s “Thymidine Kinase 2 Deficiency Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Thymidine Kinase 2 Deficiency, historical and forecasted epidemiology, as well as the Thymidine Kinase 2 Deficiency Market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Thymidine Kinase 2 Deficiency Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Thymidine Kinase 2 Deficiency Market Size from 2020 to 2034. The report also covers Thymidine Kinase 2 Deficiency Treatment practices and unmet medical needs to curate the best opportunities and assess the market’s potential.
Scope of the Thymidine Kinase 2 Deficiency Market Report | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Thymidine Kinase 2 Deficiency Epidemiology |
Segmented by:
|
|
Thymidine Kinase 2 Deficiency Drugs Market |
Segmented by:
|
|
Thymidine Kinase 2 Deficiency Market Analysis |
|
Thymidine Kinase 2 Deficiency Disease Understanding
Thymidine Kinase 2 Deficiency Overview
Thymidine Kinase 2 deficiency is a rare autosomal recessive disorder caused by mutations in the TK2 gene, which is crucial for mitochondrial DNA (mtDNA) maintenance. This condition leads to a significant reduction in mtDNA, resulting in progressive muscle weakness and various systemic complications. Patients often present with symptoms such as weakness in the limbs, respiratory difficulties, and issues with eye movement and swallowing. The severity and progression of the disease can vary widely among individuals, with some experiencing early onset and rapid decline, while others may have a later onset with a slower progression.
Thymidine Kinase 2 Deficiency Diagnosis
Diagnosis of TK2d typically involves a combination of genetic testing and clinical evaluations. Genetic testing is conducted to identify mutations in the TK2 gene, confirming the diagnosis. In addition to genetic analysis, clinicians may perform muscle biopsies to assess for signs of mitochondrial myopathies, such as ragged red fibers or abnormal mitochondria under microscopic examination. The comprehensive evaluation of symptoms alongside these diagnostic methods helps to establish a clear diagnosis and differentiate TK2d from other mitochondrial disorders.
Thymidine Kinase 2 Deficiency Treatment
Currently, there are no globally approved Thymidine Kinase 2 Deficiency therapies that specifically target TK2d, leaving treatment primarily focused on symptom management through a multidisciplinary approach. This care strategy aims to alleviate complications and enhance the quality of life for affected individuals. Neurological support often involves tailored physical and occupational therapy to address progressive muscle weakness and improve mobility. For patients experiencing respiratory insufficiency, non-invasive or invasive ventilatory support is crucial to maintain adequate oxygenation and prevent respiratory failure. Nutritional management is another critical aspect, as high-calorie diets and, in severe cases, gastrostomy feeding are often required to counter malnutrition and ensure sufficient energy intake. Additionally, mobility aids such as wheelchairs are frequently necessary for individuals with advanced muscle weakness, enabling greater independence and reducing the burden of physical limitations.
Thymidine Kinase 2 Deficiency Epidemiology
The Thymidine Kinase 2 deficiency epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Thymidine Kinase 2 Deficiency Prevalence Cases, Total Thymidine Kinase 2 Deficiency Diagnosed Prevalent Cases, Thymidine Kinase 2 Deficiency Gender-specific Cases, Thymidine Kinase 2 Deficiency Phenotype-specific Cases, and Thymidine Kinase 2 Deficiency Treated Cases in the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from Thymidine Kinase 2 Deficiency Epidemiological Analyses and Forecast
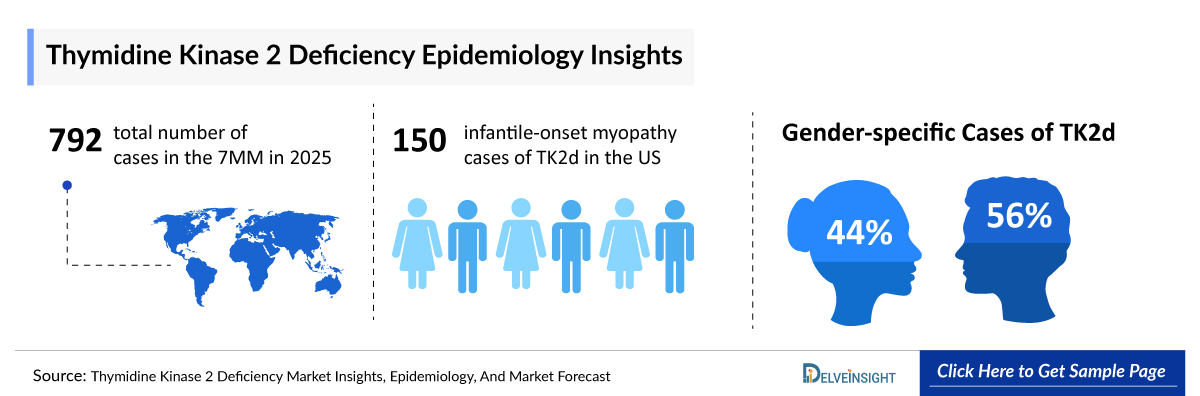
- Among the 7MM, the United States accounted for the highest number of cases of Thymidine Kinase 2 deficiency in 2023, with nearly 560 cases. These cases are anticipated to increase by 2034.
- In 2023, the United States reported the highest number of infantile-onset myopathy cases of TK2d, with ~150 cases, followed by childhood-onset myopathy and late-onset myopathy cases, with ~140 and ~60 cases, respectively.
- In 2023, Gender-specific cases of TK2d in the United States were ~160 and ~200 for women and men, respectively.
- Among EU4 and the UK, Germany accounted for the highest number of total prevalent cases in 2023, with approximately 150 cases.
Thymidine Kinase 2 Deficiency Epidemiology Segmentation
- Total Thymidine Kinase 2 Deficiency Prevalence Cases
- Total Thymidine Kinase 2 Deficiency Diagnosed Prevalent Cases
- Thymidine Kinase 2 Deficiency Gender-specific Cases,
- Thymidine Kinase 2 Deficiency Phenotype-specific Cases
- Thymidine Kinase 2 Deficiency Treated Cases
Thymidine Kinase 2 Deficiency Drugs Analysis
The section dedicated to drugs in the Thymidine Kinase 2 Deficiency treatment market report provides an in-depth evaluation of late-stage (Phase II) drugs related to Thymidine Kinase 2 deficiency pipeline drugs analysis. The drug chapters section provides valuable information on various aspects related to Thymidine Kinase 2 deficiency Clinical Trials, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting Thymidine Kinase 2 deficiency.
Thymidine Kinase 2 Deficiency Emerging Therapies
MT1621 (Doxecitine and Doxribtimine): UCB Biosciences
Doxecitine and doxribtimine (MT1621) is a fixed-dose combination therapy that targets the underlying pathophysiology of Thymidine Kinase 2 Deficiency by restoring mitochondrial DNA (mtDNA) replication fidelity. Doxecitine and doxribtimine consist of a combination of deoxynucleosides (the building blocks of mtDNA) given orally. Deoxynucleoside combination therapy improves nucleotide balance, increases mtDNA copy number, improves cell function, and prolongs life in preclinical models of TK2d. By increasing the levels of thymidine and deoxycytidine in the body, the medicine is expected to make up for the deficiencies in TK2 activity, thereby improving the production of mitochondrial DNA and helping relieve the patient’s symptoms.
Doxecitine and doxribtimine are in clinical development for the treatment of TK2d. In the pivotal phase II trial (NCT03845712), doxecitine and doxribtimine are administered orally up to a maximum of 800 mg/kg/day (400 mg/kg/day of dC and 400 mg/kg/day of dT) as tolerated. In February 2019, the FDA granted Breakthrough Therapy designation, MT1621 has also been granted PRIME designation by the EMA and Orphan Drug Designation (ODD) by both the FDA and EMA in 2018.
Thymidine Kinase 2 Deficiency Market Outlook
Although there are no FDA-approved drugs specifically for TK2d, treatment primarily centers on deoxynucleoside therapy, which provides the essential building blocks needed for mitochondrial DNA replication and maintenance. This therapy has shown promise in clinical trials, demonstrating improvements in muscle strength and respiratory function with minimal side effects. By bypassing the enzymatic deficiency caused by mutations in the TK2 gene, deoxynucleoside therapy offers hope for better management of this challenging condition. Ongoing research continues to explore additional therapeutic strategies to further enhance patient outcomes and quality of life.
In a nutshell, not many potential therapies are being investigated to manage Thymidine Kinase 2 deficiency. Even though it is too soon to comment on the above-mentioned promising candidate to enter the market during the forecast period (2024–2034). Eventually, this drug will create a significant difference in the landscape of Thymidine Kinase 2 deficiency in the coming years. The treatment space is expected to experience a significant positive shift in the coming years, owing to the improvement in healthcare spending worldwide.
- The Thymidine Kinase 2 Deficiency Market Size in the 7MM was USD 5 Million in 2025 and is projected to increase during the forecast period (2024–2034).
- In the total Thymidine Kinase 2 Deficiency Market Size in the 7MM, the United States accounted for the highest market share, i.e., approximately 60% in 2023, followed by Germany.
- Among EU4 and the UK, Germany accounted for almost 9% of the market size in 2023.
- Currently, only the Standard of Care (SOC) is being used as the only option of therapy for the management of Thymidine Kinase 2 deficiency.
- The leading Thymidine Kinase 2 Deficiency Companies, such as UCB, and others.
Thymidine Kinase 2 Deficiency Drugs Uptake
This section focuses on the uptake rate of potential Thymidine Kinase 2 Deficiency drugs expected to be launched in the market during 2020–2034.
Thymidine Kinase 2 Deficiency Pipeline Development Activities
The Thymidine Kinase 2 Deficiency Therapeutics Market Report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Thymidine Kinase 2 Deficiency Companies involved in developing targeted therapeutics.
Latest KOL Views on Thymidine Kinase 2 Deficiency
To stay abreast of the latest trends in the Thymidine Kinase 2 Deficiency market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research. We have reached out to industry experts to gather insights on various aspects of Thymidine Kinase 2 deficiency, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 10 KOLs across the 7MM. We contacted institutions such as the Meyer Hospital, Instituto de Biomedicina de Sevilla, Centre for Biomedical Network Research on Rare Diseases (CIBERER), etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Thymidine Kinase 2 deficiency market, which will assist our clients in analyzing the overall epidemiology and Thymidine Kinase 2 Deficiency market scenario.
Thymidine Kinase 2 Deficiency Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in trials for Thymidine Kinase 2 deficiency, one of the most important primary endpoints was achieving the number of participants who experience Adverse Events (AEs), etc. Based on these, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Thymidine Kinase 2 Deficiency Market Access and Reimbursement
The Thymidine Kinase 2 Deficiency market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Because newly authorized drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the Thymidine Kinase 2 Deficiency market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
Thymidine Kinase 2 Deficiency Market Report Insights
- Patient-based Thymidine Kinase 2 Deficiency Market Forecasting
- Therapeutic Approaches
- Thymidine Kinase 2 Deficiency Market Size
- Thymidine Kinase 2 Deficiency Market Trends
- Existing Thymidine Kinase 2 Deficiency Drugs Market Opportunity
Thymidine Kinase 2 Deficiency Market Report Key Strengths
- 11-year Thymidine Kinase 2 Deficiency Market Forecast
- The 7MM Coverage
- Thymidine Kinase 2 Deficiency Epidemiology Segmentation
- Key Cross Competition
Thymidine Kinase 2 Deficiency Market Report Assessment
- Current Thymidine Kinase 2 Deficiency Treatment Market Practices
- Reimbursements
- Thymidine Kinase 2 Deficiency Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet Needs)
Key Questions Answered in the Thymidine Kinase 2 Deficiency Market Report
Thymidine Kinase 2 Deficiency Market Insights
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Thymidine Kinase 2 deficiency management recommendations?
- Would research and development advances pave the way for future tests and therapies for Thymidine Kinase 2 deficiency?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of Thymidine Kinase 2 deficiency?
- What kind of uptake will the new therapies witness in the coming years in Thymidine Kinase 2 deficiency patients?
Stay updated with us for Recent Articles


-pipeline.png&w=256&q=75)

