TL1A Binder Market Forecast
DelveInsight’s “TL1A Binder Market Size, Target Population, Competitive Landscape and Market Forecast - 2034” report delivers an in-depth understanding of TL1A Binder, addressable patient pool, competitive landscape, and future market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan.
The TL1A Binder market report provides insights around existing treatment practices in patients with TL1A Binder, approved (if any) and emerging TL1A Binder, market share of individual therapies, patient pool eligible for treatment with TL1A Binder, along with current and forecasted 7MM TL1A Binder market size from 2020-2034 by therapies and by indication. The report also covers current unmet needs and challenges while incorporating new classes in treatment paradigm, variations in accessibility and acceptability of new TL1A Binder in different geographies, along with insights on TL1A Binder pricing reimbursements to curate the best opportunities and assess the market’s potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain), and the UK
- Japan
Study Period: 2020–2034
TL1A Binder Overview
This segment will provide detailed information beginning with the inhibitor journey from discovery of the mutation or protein expression to its entry into clinical development followed by its upcoming commercial potential. This segment will dive into the different indications for which the inhibitor is being developed for, which would further give insights on the potential addressable patient population. Moreover, this segment will also give a brief overview around the existing treatment paradigm of the target indications.
Key Factors Driving the TL1A Binder Market
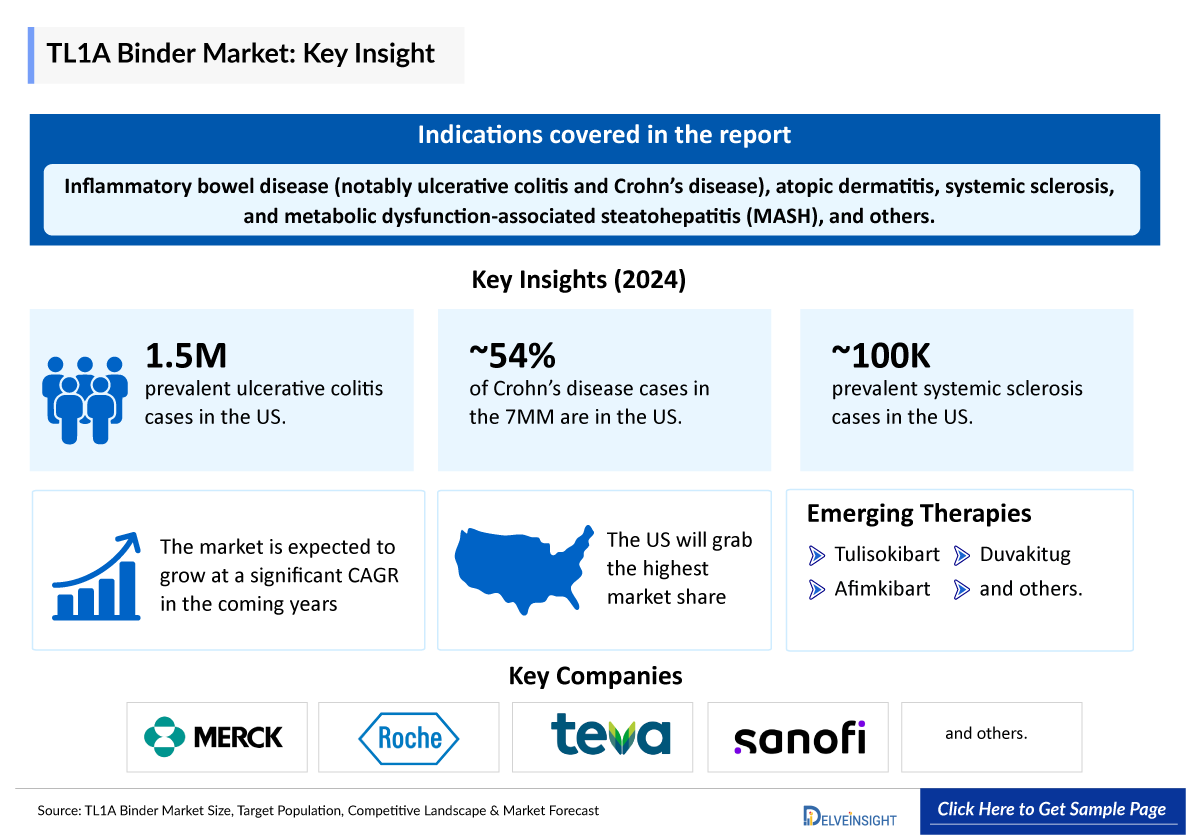
Rising Prevalence of Inflammatory Bowel Disease (IBD)
The global incidence of IBD, encompassing Crohn's disease and ulcerative colitis, is on the rise. The total diagnosed prevalent cases of Crohn’s disease in the United States were around 1.1 million cases in 2024. On the other hand, the total ulcerative colitis diagnosed prevalent cases in the 7MM comprised approximately 3.1 million cases in 2024. These cases are further expected to increase by 2034. This surge is attributed to factors such as lifestyle changes and environmental influences. As a result, there is an increasing demand for effective treatments targeting the underlying mechanisms of these conditions.
Strategic Collaborations and Investments
Pharmaceutical companies are increasingly investing in TL1A-targeted therapies. Merck's acquisition of Prometheus Biosciences for $10.8 billion underscores the potential of TL1A as a therapeutic target. Similarly, Teva and Sanofi's collaboration on duvakitug highlights the growing interest and commitment to developing TL1A-based treatments.
Expanding Therapeutic Applications
While TL1A-targeted therapies are primarily being developed for IBD, their potential applications extend to other diseases such as Systemic Sclerosis, Atopic Dermatitis, MASH, and others. This broadens the market scope and increases the commercial appeal of TL1A-targeted treatments.
Launch of Emerging TL1A-targeted Therapies
The TL1A binder pipeline is rapidly gaining momentum, with competition intensifying among major players including Merck’s tulisokibart (MK-7240), Roche’s afimkibart (RG6631), and Teva/Sanofi’s duvakitug (TEV’574/SAR447189), all progressing through various immune-mediated indications.
TL1A Binder in Clinical Practice
This section will give in depth information about the existing local and systemic options in the current treatment paradigm of all the potential indications, in which most of the pharmaceutical companies are actively evaluating their inhibitors. Potential of the emerging TL1A Binder in changing the current clinical practice guidelines is crucial to analyze especially when it comes to real world scenario.
It will also include the relevance and importance of incorporation of biomarker testing at varying stages of the disease. It is also important to understand that implementing such tests in routine clinical practice is not uniform in different countries due to issues such as cost, accessibility, reimbursement and non-recommendation in guidelines.
TL1A Binder Drug Chapters
The drug chapter segment of the TL1A Binder report encloses a detailed analysis of marketed therapies and late-stage (Phase III and Phase II) therapies. It also helps understand the TL1A Binder clinical trial details, pharmacological action, agreements and collaborations related to TL1A Binder, their approval timelines, patent details, advantages and disadvantages, latest news and press releases.
TL1A Binder Marketed Drugs
The TL1A Binder marketed drug section will provide detailed drug profiles of already approved therapies. Information around clinical development activities, launch timing, regulatory milestones along with safety and efficacy data of the therapy will be included.
TL1A Binder Emerging Drugs
Apart from a comprehensive TL1A Binder competitive landscape in tabular form, the emerging TL1A Binder chapters provides the product details and other development activities of the emerging TL1A Binder under the late and mid-stage of clinical development for various indications.
Note: Detailed list will be provided in the final report.
Drug Class Insights
The Drug Class Insights section will provide comprehensive information on TL1A Binder as a class. This will include a broad overview of the class and its role in treating specific conditions. Insights may cover the historical clinical development of TL1A Binder, their mechanism of action, their subtypes and future commercial prospects. Additionally, the section will provide detailed information about current trends, challenges, and future prospects for this class of drugs.
TL1A Binder Market Outlook
This section will include details on changing TL1A Binder market dynamics post initiation of clinical development activities of the inhibitor. It will also provide a detailed summary and comparison of all the therapies being developed by leading players in this space. This section will highlight the advantages of one therapy over the other after assessment based on parameters such as data availability in the form of safety and efficacy, number of patients enrolled in each trial, and trial’s inclusion criteria. There will be a Key focus on the importance of development and need for the commercial success of these targeted therapies to achieve treatment goals that physicians and patients are looking for. It will also sum up all the early stage players active in this space.
TL1A Binder Drugs Uptake
This section focuses on the uptake rate of potential TL1A Binder already launched and expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, efficacy data, and order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
TL1A Binder Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
TL1A Binder Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for TL1A Binder.
KOL Views
To keep up with current and future market trends, we incorporate Key physicians, Therapy Area Researcher’s, and other Industry Experts’ opinions working in the domain through primary research to fill in the data gaps and validate our secondary research. 25+ Key Opinion Leaders (KOLs) were contacted for insights on TL1A Binder’ incorporation in the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along with challenges related to accessibility.
Qualitative Analysis
We perform qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Analyst views. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the cost analysis and existing and evolving treatment landscape.
Market Access and Reimbursement
This section will include insights around the standard HTA pricing, recent reformations in 2024 and modifications in reimbursement process in the 7MM. For example, In the United States, a multi payer model exists when it comes to drug pricing regime, which is currently undergoing significant changes, with recent federal legislation, such as the Prescription Drug Pricing Reform provisions of the Inflation Reduction Act, significantly altering the pricing regime under certain federal programs. Whereas in Germany, the market access differs from the systems followed in many other countries as no pricing and reimbursement approval is required during launch of a new therapy.
Moreover, this section will also provide details on reimbursement of approved therapy, if any.
Scope of the Report
- The report covers a segment of key events, an executive summary, target patient pool, epidemiology and market forecasts, information around patient journey and varying biomarker testing rates
- Additionally, an all-inclusive account of the current and emerging therapies drug chapters, insights on TL1A Binder addressable patient pool
- A detailed review of the TL1A Binder market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, and treatment preferences that help in shaping and driving the 7MM TL1A Binder market.
- Market Size of Inhibitors by therapies and indication will be provided
TL1A Binder Report Key Strengths
- 11 Years TL1A Binder Market Forecast
- The 7MM Coverage
- TL1A Binder Competitive Landscape of current and emerging therapies
- TL1A Binder Total Addressable patient population
- Drugs Uptake and Key Market Forecast Assumptions
- Approved and Emerging therapy Profiles
- Physician’s perspectives/KOL opinions
- Biomarker testing and Patient journey
- Qualitative Analysis (SWOT and Analyst Views)
- TL1A Binder Market Size by therapy and indication
- Existing and Future Market Opportunity
- Unmet Needs
FAQs
- What was the TL1A Binder total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for TL1A Binder market growth?
- Which TL1A Binder is going to be the largest contributor by 2034?
- What is the market access and reimbursement scenario of TL1A Binder?
- What are the pricing variations among different geographies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
Reasons to Buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the TL1A Binder Market.
- Understand the existing TL1A Binder market opportunities and future trends in varying geographies
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility and acceptability of emerging treatment options along with unmet need of current therapies
- Details on report methodology, top indications covered, market assumptions, patient journey and KOLs to strengthen the pharmaceutical companies’ development and launch strategy.