Traveler's Diarrhea Market Summary
- The Traveler's Diarrhea Market Size is anticipated to grow with a significant CAGR duirng the study period (2020-2034).
- The Traveler's Diarrhea companies developing therapies in the treatment market include - Cosmo Pharmaceuticals, RedHill Biopharma, and others.
Traveler's Diarrhea Market and Epidemiology Analysis
- Travelers' diarrhea is the most common travel-related illness, approximately 40 to 60% of travelers suffer from the indication.
- While travelers' diarrhea can affect individuals of any age, the elderly and children under the age of four face the highest risk of developing complications.
- Bacterial, viral, and parasitic infections can cause symptoms, though bacterial sources represent the most frequent etiology.
- Typically, treatment involves fluid replacement to prevent dehydration, taking over-the-counter (OTC) options such as loperamide and bismuth subsalicylate are also considered.
- The most commonly used antibiotics for travelers’ diarrhea are azithromycin and nonabsorbable rifaximin.
- In more severe cases where symptoms persist, a person may require medications that target the causative agent such as AEMCOLO for the treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli in adults.
- Currently, the development of novel therapeutics specifically targeting the pathogens responsible for travelers' diarrhea, could address the need for more effective treatment options, particularly in cases of severe or antibiotic-resistant infections.
- In January 2025, Immuron Limited announced the submission of a Clinical Study Report to the FDA for Phase 2 trials of Travelan®, aimed at mitigating travelers’ diarrhea. The study showed significant immunological and microbiome responses, suggesting benefits in reducing and clearing pathological ETEC bacteria and improving gut health.
Request for unlocking the Sample Page of the "Traveler's Diarrhea Market Insights"
Key Factors Driving the Traveler's Diarrhea Market
-
Rising International Travel and Tourism: The steady growth in global tourism, business travel, and adventure travel particularly to high-risk regions in Asia, Africa, and Latin America continues to increase the incidence of traveler’s diarrhea, directly driving demand for preventive and therapeutic products.
-
Increasing Awareness of Travel Health and Preventive Care: Greater awareness among travelers about food- and water-borne infections, supported by travel clinics and pre-travel consultations, has boosted the uptake of prophylactic medications, vaccines, probiotics, and travel health kits.
-
Availability of Effective Pharmacological Treatments: The widespread use of antibiotics (such as rifaximin and azithromycin), antidiarrheals, and oral rehydration solutions has improved disease management, supporting consistent market demand across both prescription and OTC segments.
-
Expansion of OTC and Retail Pharmacy Channels: Easy access to over-the-counter medications through retail and online pharmacies has enhanced product availability and convenience, encouraging early treatment and self-management among travelers.
-
Pipeline Development and Preventive Innovations: Ongoing R&D efforts focused on vaccines, non-systemic antibiotics, microbiome-based therapies, and improved formulations are expected to strengthen long-term market growth and address unmet needs such as antimicrobial resistance.
DelveInsight's “Traveler's Diarrhea Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Traveler's Diarrhea, historical and forecasted epidemiology as well as the Traveler's Diarrhea therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Traveler's Diarrhea market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Traveler's Diarrhea market size from 2020 to 2034. The report also covers current Traveler's Diarrhea treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Scope of the Traveler's Diarrhea Market | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Traveler's Diarrhea Market |
|
|
Traveler's Diarrhea Market Size | |
|
Traveler's Diarrhea Companies |
Cosmo Pharmaceuticals, RedHill Biopharma, and others |
|
Traveler's Diarrhea Epidemiology Segmentation |
|
Traveler's Diarrhea Understanding
Traveler's Diarrhea Overview, Country-Specific Treatment Guidelines and Diagnosis
Traveler’s diarrhea refers to a common travel-related illness. It typically describes a gastrointestinal infection after consuming contaminated food or water in an area that is not local to the person traveling. Diarrhea is characterized by abnormally loose or watery stools. Health experts may define a Traveler’s diarrhea as 3 or more loose stools in a 24-hour period during a trip abroad to a country with different hygiene practices. It often presents with other symptoms, such as nausea and vomiting. The onset of symptoms may be during or within 10 days of travel and typically lasts for 3–5 days.
Less severe symptoms include nausea, vomiting, fever, bloating, excessive gas, loss of appetite, and an urgent need to defecate, whereas these symptoms can also become severe which will indicate an urgency to immediately consult a health practitioner and severe symptoms include: severe, intolerable pain in the abdomen or rectum, persistent vomiting for more than four hours, resulting in the inability to keep liquids down, fever higher than 102˚F, bloody stools, symptoms of dehydration.
The Travelers' Diarrhea report provides an overview of Travelers' Diarrhea pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report..
Travelers' Diarrhea Treatment
In many cases, symptoms of Travelers' Diarrhea resolve in a few days. As such, treatment often involves fluid replacement to avoid dehydration. This can include drinking plenty of fluids such as clear broths and beverages rich in electrolytes. Other treatment options include antimotility agents (loperamide), Antisecretory/anti-inflammatory agents (bismuth subsalicylate), antibiotic agents (azithromycin, cefixime, ciprofloxacin), trimethoprim/sulfamethoxazole, and others, which may also help to reduce the severity and duration of symptoms and lessen the interruption to travel plans.
In 2018, AEMCOLO was approved by the US FDA for the treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli in adults.
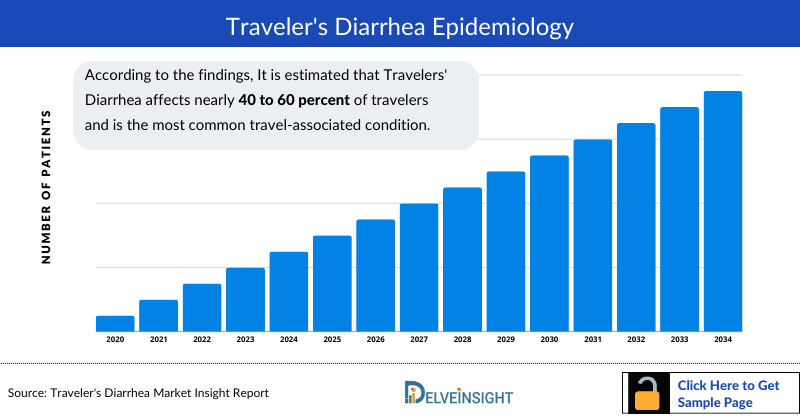
Travelers' Diarrhea Epidemiology
The Travelers' Diarrhea epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Travelers' Diarrhea epidemiology is segmented with detailed insights into Total Diagnosed Cases of Travelers' Diarrhea, Age-specific Cases, and total treated cases of Travelers' Diarrhea.
Key Findings from Travelers' Diarrhea Epidemiological Analyses and Forecast
- According to the findings, It is estimated that Travelers' Diarrhea affects nearly 40 to 60 percent of travelers and is the most common travel-associated condition.
- Children less than four years of age are at particularly high risk of acquiring travelers’ diarrhea and of suffering subsequent dehydration.
Travelers' Diarrhea Eidemiology Segmentation
- By Geography
- By Population Type cases of Travelers' Diarrhea
- By Age-specific cases of Travelers' Diarrhea
- By Etiology-specific cases of Travelers' Diarrhea
Recent Developments in the Travelers' Diarrhea Treatment Landscape
- In January 2025, Immuron Limited announced the submission of a Clinical Study Report to the FDA for Phase 2 trials of Travelan®, aimed at mitigating travelers’ diarrhea. The study showed significant immunological and microbiome responses, suggesting benefits in reducing and clearing pathological ETEC bacteria and improving gut health.
Travelers' Diarrhea Drug Analysis
The drug chapter segment of the Travelers' Diarrhea report encloses a detailed analysis of Travelers' Diarrhea marketed drugs and late-stage (Phase III and Phase II) Travelers' Diarrhea pipeline drugs. It also deep dives into the Travelers' Diarrhea pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Traveler's Diarrhea Marketed Drugs
AEMCOLO: Cosmo Pharmaceuticals/ RedHill Biopharma
AEMCOLO is a rifamycin antibacterial indicated for the treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli in adults. AEMCOLO is not recommended for use in patients with diarrhea complicated by fever and/or bloody stool or due to pathogens other than noninvasive strains of E. coli. It is approved in the US and EU. In the US it is currently licensed to Cosmo’s partner RedHill Biopharma. In September 2023, Cosmo announced the termination by mutual agreement of the license with Dr. Falk Pharma for Rifamycin SV MMX in the EU and other selective countries and announced the signing of a new license and supply agreement with Adalvo in Europe, Asia-Pacific (APAC), Middle East/North Africa (MENA) and Latin America (LATAM) regions.
Note: Detailed current therapies assessment will be provided in the full report of Travelers' Diarrhea...
Traveler's Diarrhea Emerging Drugs
TRAVELAN (IMM-124E): Immuron
TRAVELAN is a highly purified preparation of highly specific antibodies that target pathogenic bacteria and the toxins they produce. It does not react with or alter the normal microbial community of a healthy gastrointestinal tract, unlike antibiotics. TRAVELAN helps to support a healthy digestive system by supporting the gut’s immune defenses, whether one’s traveling or at home. Currently, it is in Phase II of its clinical development for the treatment of Travelers' Diarrhea.
The company is planning to conduct a Phase III trial soon for the treatment of Travelers' Diarrhea.
Note: Detailed emerging therapies assessment will be provided in the final report....
Travelers' Diarrhea Market Outlook
Key players, such as Immuron and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Travelers' Diarrhea.
The global Traveler’s Diarrhea market is projected to grow steadily over the coming decade, driven by rising international tourism, increased awareness of travel-related health risks, and broader access to treatment options. Growth is supported by strong demand for effective therapies (such as antibiotics, antidiarrheals, rehydration products, and emerging preventive measures like vaccines and probiotics), expanding travel into high-risk regions with sanitation challenges, and technological advances in diagnostics and digital health tools.
North America currently holds significant market share due to high outbound travel and healthcare awareness, while the Asia-Pacific region is one of the fastest-growing markets due to increasing travel activity and rising healthcare access. Persistent challenges include antimicrobial resistance and the need for expanded preventive solutions, but overall the outlook remains positive as travel volumes and health preparedness continue to increase.
Travelers' Diarrhea Competitive Landscape
The Traveler’s Diarrhea competitive landscape is moderately competitive and evolving, characterized by a mix of large multinational pharmaceutical companies, specialized gastroenterology firms, and emerging biotech players. Major established players include Bausch Health Companies Inc. (Salix Pharmaceuticals) with widely used products like rifaximin, Johnson & Johnson, Pfizer Inc., Sanofi SA, GlaxoSmithKline plc, and Teva Pharmaceutical Industries Ltd., which offer a range of OTC and prescription treatments targeting symptom relief and infection control. Other significant participants include Immuron Limited, Valneva SE (focused on vaccine development), and regional manufacturers that contribute through probiotics, combination therapies, and supportive care products like oral rehydration solutions.
Competitive dynamics emphasize innovation (e.g., delayed-release antibiotics and preventive vaccines), strategic collaborations, product diversification, pricing strategies, and expanding distribution channels such as retail and online pharmacies. The presence of generic manufacturers also maintains pricing pressure, pushing incumbents toward differentiation through R&D and novel formulations.
Key Travelers' Diarrhea Companies
The Key Traveler's Diarrhea companies actively involved in the Traveler's Diarrhea treatment landscape include -
- Cosmo Pharmaceuticals
- RedHill Biopharma, and others
Travelers' Diarrhea Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Travelers' Diarrhea Clinical Trial Activities
The Traveler's Diarrhea pipeline report provides insights into different Traveler's Diarrhea clinical trials within Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Traveler's Diarrhea Pipeline Development Activities
The Traveler's Diarrhea clinical trial analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Travelers' Diarrhea emerging therapies.
Latest KOL Views on Traveler's Diarrhea Market Report
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of Travelers' Diarrhea. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Traveler's Diarrhea Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Traveler's Diarrhea Market Access and Reimbursement
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Traveler's Diarrhea Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Travelers' Diarrhea, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Travelers' Diarrhea market, historical and forecast market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Travelers' Diarrhea market.
Travelers' Diarrhea Market Report Insights
- Traveler's Diarrhea Patient Population
- Traveler's Diarrhea Therapeutic Approaches
- Travelers' Diarrhea Pipeline Analysis
- Travelers' Diarrhea Market Size and Trends
- Existing and future Market Opportunity
Travelers' Diarrhea Market Report Key Strengths
- Ten Years Forecast
- 7MM Coverage
- Travelers' Diarrhea Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Traveler's Diarrhea Drugs Uptake
- Key Traveler's Diarrhea Market Forecast Assumptions
Travelers' Diarrhea Market Report Assessment
- Current Traveler's Diarrhea Treatment Practices
- Traveler's Diarrhea Unmet Needs
- Traveler's Diarrhea Pipeline Product Profiles
- Traveler's Diarrhea Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Traveler's Diarrhea Market Drivers
- Traveler's Diarrhea Market Barriers
FAQs Related to the Traveler's Diarrhea Market Report:
- What is the growth rate of the 7MM Travelers' Diarrhea treatment market?
- What was the Travelers' Diarrhea total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of Travelers' Diarrhea?
- How many companies are developing therapies for the treatment of Travelers' Diarrhea?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy Traveler's Diarrhea Market Forecast Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Travelers' Diarrhea Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.