Vitreomacular Traction Syndrome Market
- The market for Vitreomacular Traction Syndrome Marekt is poised for growth during the forecast period (2024-2034), due to diagnostic technologies, an aging population, advancements in surgical techniques, and ongoing research into new treatments.
- Epidemiological data indicate that Vitreomacular Traction Syndrome is a common retinal condition, particularly among the aging population. The increasing prevalence with age underscores the importance of early detection and management, especially considering the potential impact on vision and quality of life.
- Key challenges in Vitreomacular Traction Syndrome management include diagnostic complexities due to variable presentations, limited treatment options, and the delicate balance between intervention benefits and risks, underscoring the critical need for comprehensive strategies and ongoing research advancements.
- The treatment panorama for Vitreomacular Traction Syndrome is predominantly defined by Ocriplasmin (JETREA) as the sole approved therapy. Beyond this, the therapeutic landscape remains devoid of approved options, with a sparse emerging pipeline, underscoring unmet medical needs in this domain.
Request for unlocking the CAGR of the "Vitreomacular Traction Syndrome Drug Market"
DelveInsight’s comprehensive report titled “Vitreomacular Traction Syndrome Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Vitreomacular Traction Syndrome. The report presents historical and projected epidemiological data covering Total Prevalent Cases of Vitreomacular Traction Syndrome, Age-Specific Prevalent Cases Of Vitreomacular Traction Syndrome, Gender-specific Diagnosed Prevalent Cases of Vitreomacular Traction Syndrome, and Symptomatic Cases Of Vitreomacular Traction Syndrome. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing treatment practices and unmet medical requirements in Vitreomacular Traction Syndrome. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Vitreomacular Traction Syndrome Epidemiology |
|
|
Vitreomacular Traction Syndrome Market |
|
|
Market Analysis |
|
|
Vitreomacular Traction Syndrome Market Players |
|
|
Challenges |
Challenges associated with Vitreomacular Traction Syndrome include the potential for vision loss if left untreated, the difficulty in diagnosing the condition, the need for specialized diagnostic tests like Optical Coherence Tomography (OCT) and Fluorescein angiography, and the variability in symptoms which can overlap with other eye diseases, necessitating a thorough evaluation by an ophthalmologist. Additionally, the decision-making process for treatment can be complex, as some cases may resolve on their own while others may require surgery or medication, leading to the challenge of determining the most appropriate course of action based on the severity of the condition and individual patient factors. |
Vitreomacular Traction Syndrome Overview
Vitreomacular Traction Syndrome is a retinal disorder characterized by abnormal adhesion between the vitreous gel and the macula, a part of the retina responsible for central vision. The causes of VMT are not fully understood, but it often occurs due to age-related changes in the vitreous gel composition, leading to its abnormal adherence to the macula. Risk factors for Vitreomacular traction syndrome include advanced age, previous eye surgery or trauma, and certain eye conditions such as diabetic retinopathy or macular degeneration.
Symptoms of VMT may include distorted or blurred central vision, difficulty reading or seeing fine details, and in some cases, a sensation of pulling or tugging in the affected eye. These symptoms can significantly impair visual function and quality of life, particularly in activities that require clear central vision such as reading, driving, or recognizing faces. Additionally, Vitreomacular traction syndrome may progress to more severe complications such as macular hole formation, further exacerbating visual impairment and diminishing overall well-being.
Vitreomacular Traction Syndrome Diagnosis and Treatment Algorithm
Diagnosing Vitreomacular Traction Syndrome demands meticulous evaluation of retinal imaging and clinical symptoms. Optical coherence tomography (OCT) serves as a cornerstone in this process, offering detailed insights into the vitreous-macular interface. However, VMT diagnosis is intricate due to variable symptomatology and potential overlap with other retinal disorders like diabetic macular edema. Furthermore, interpreting OCT findings necessitates expertise to discern subtle changes indicative of VMT.
The complexity of Vitreomacular traction syndrome diagnosis is compounded by concurrent retinal pathologies and the challenge of distinguishing clinically significant VMT from benign vitreomacular adhesion. This demands a collaborative approach between ophthalmologists and retinal specialists to navigate the nuances of VMT presentation accurately. Close scrutiny of OCT images and meticulous clinical assessment are imperative to ensure precise diagnosis.
Treating Vitreomacular Traction Syndrome (VMT) aims to alleviate symptoms and prevent progression to more severe complications. The primary treatment option for symptomatic VMT is vitrectomy, a surgical procedure where the vitreous gel is removed to release traction on the macula. Vitrectomy is often accompanied by membrane peeling to further relieve traction and improve visual outcomes. In cases where vitrectomy is not feasible or warranted, observation may be recommended, especially for asymptomatic or mild cases.
However, treating VMT poses several challenges. Firstly, vitrectomy is an invasive procedure associated with potential risks, including retinal detachment, cataract formation, and infection. Additionally, not all patients with VMT experience significant visual impairment or warrant surgical intervention, making the decision to proceed with treatment complex. Furthermore, there is a lack of pharmacological options specifically approved for VMT, leaving patients with limited non-surgical alternatives. This underscores the need for careful patient selection and shared decision-making between patients and ophthalmologists to optimize treatment outcomes while minimizing risks.
Vitreomacular Traction Syndrome Epidemiology
The epidemiology section on the Vitreomacular Traction Syndrome market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The prevalent patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of Vitreomacular Traction Syndrome. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
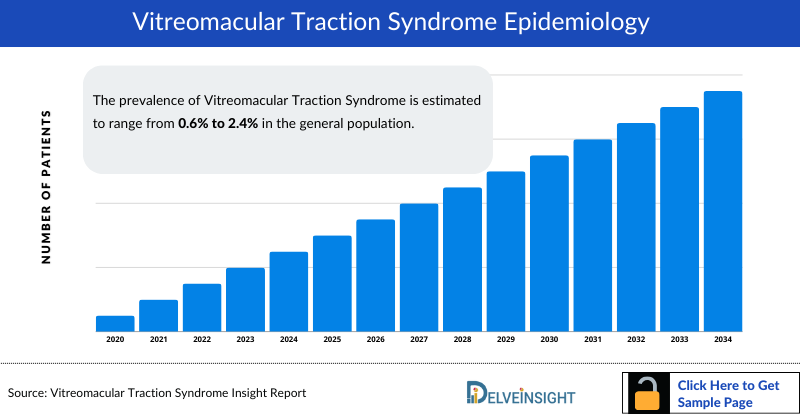
The prevalence of Vitreomacular Traction Syndrome is estimated to range from 0.6% to 2.4% in the general population. However, prevalence rates may vary depending on factors such as age, gender, and underlying ocular conditions.
As per our analysis, Vitreomacular Traction Syndrome prevalence appears higher in females. This trend aligns with research suggesting gender differences in ocular health, potentially influenced by hormonal factors and healthcare-seeking behaviors.
The prevalence of Vitreomacular Traction Syndrome tends to increase with age. As such, Vitreomacular Traction Syndrome is more commonly observed in older adults, particularly those over the age of 50 or 60 years. However, it's essential to consider that Vitreomacular Traction Syndrome can also occur in younger individuals, albeit less frequently, highlighting the multifactorial nature of its epidemiology.
Vitreomacular Traction Syndrome Market Outlook
The Vitreomacular Traction Syndrome market outlook presents opportunities and challenges. Ocriplasmin (JETREA), a recombinant truncated form of human plasmin, is a pharmacologic option for treating Vitreomacular Traction (VMT) syndrome. The drug is injected into the vitreous through a self-sealing microincision in the wall of the eye (“intravitreal injection”). It has been approved in Europe in 2013 for the treatment of VMT and by the FDA in the US in 2012 for the treatment of symptomatic VMA. With the approval of Ocriplasmin (JETREA) as the primary therapy, the market initially showed promise. However, the limited efficacy and safety concerns associated with Ocriplasmin underscore the need for alternative treatments. Despite this, the current treatment landscape lacks approved options beyond Ocriplasmin, and the emerging pipeline is sparse. This scarcity presents a significant challenge for patients and healthcare providers, as it hinders the availability of effective therapies for VMT management.
However, advancements in research and technology offer potential avenues for market growth. The development of novel pharmacological agents targeting VMT-specific pathways could address unmet medical needs and expand treatment options. Additionally, innovations in surgical techniques and devices may improve outcomes and reduce complications associated with vitrectomy, the primary surgical intervention for VMT. Furthermore, increasing awareness among healthcare professionals and patients about VMT and its impact on vision may drive demand for improved diagnostic tools and therapeutic interventions. Overall, while the VMT market currently faces obstacles, ongoing research and innovation hold promise for future advancements in VMT treatment and management.
Vitreomacular Traction Syndrome Drug Chapters
Marketed Vitreomacular Traction Syndrome Drugs
JETREA (Ocriplasmin): Oxurion/Inceptua/ThromboGenics
Jetrea (ocriplasmin) is a proteolytic enzyme indicated for the treatment of symptomatic vitreomacular adhesion and vitreomacular traction. Ocriplasmin is injected once into the vitreous body of the eyeball by an ophthalmologist (eye doctor). One injection contains 0.125 mg of the drug. After the injection, patients should be monitored for one hour to check for any side effects. Ocriplasmin has been approved in Germany since March 2013 for the treatment of vitreomacular traction (VMT) in adults, an eye disorder that is usually age-related. The drug is also an option if the condition has caused a small hole to form in the retina.
Note: Detailed marketed therapies assessment will be provided in the final report.
Emerging Vitreomacular Traction Syndrome Drugs
Currently, the Vitreomacular Traction Syndrome Emerging Pipeline is limited. However, the dynamics of Vitreomacular Traction Syndrome market size is expected to change due to improved understanding of the complex pathogenesis of Vitreomacular Traction Syndrome leading to the development of novel pharmacologic interventions during the forecast period of 2020─2034.
There is scarcity of compaanies working towards the development of new treatment therapies and some of the key players at the global level are Kato Pharmaceuticals, among others.
Resolv ER a product of Kato Pharmaceuticals is a novel drug formulation that separates the vitreous from the retina. This is useful in reducing the risk of progression for many different retinal pathologies like Diabetic retinopathy, Vitreomacular traction, Retinal tear, Retinal detachment, and Macular hole. In February 2022, FDA approved the company's investigational new drug (IND) application to conduct human studies using Resolv ER for the treatment of Vitreomacular Attachment. Currently there are no updates available about the clinical development status of Resolv ER. Additionally the website of Kato Pharmaceuticals is not assessable.
Detailed list and description will be provided in the report……
Vitreomacular Traction Syndrome Market Segmentation
DelveInsight’s ‘Vitreomacular Traction Syndrome Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Vitreomacular Traction Syndrome market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
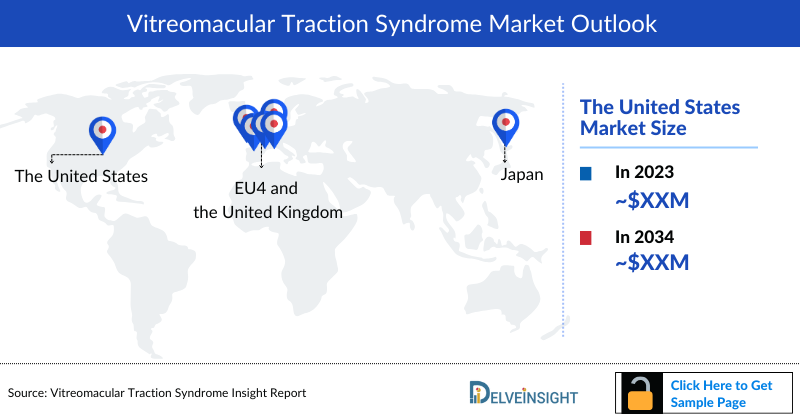
Vitreomacular Traction Syndrome Market Size by Countries
The Vitreomacular Traction Syndrome market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) Vitreomacular Traction Syndrome, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Vitreomacular Traction Syndrome Drugs Uptake
This section focuses on the sales uptake of potential Vitreomacular Traction Syndrome drugs that have recently been launched or are anticipated to be launched in the Vitreomacular Traction Syndrome market between 2020 and 2034. It estimates the market penetration of Vitreomacular Traction Syndrome drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Vitreomacular Traction Syndrome market.
The emerging Vitreomacular Traction Syndrome therapies is analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry, and other market dynamics, and the unmet need they fulfill in the Vitreomacular Traction Syndrome market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Vitreomacular Traction Syndrome.
Vitreomacular Traction Syndrome Market Access and Reimbursement
DelveInsight’s ‘Vitreomacular Traction Syndrome – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Vitreomacular Traction Syndrome. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Vitreomacular Traction Syndrome market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Vitreomacular Traction Syndrome domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Vitreomacular Traction Syndrome market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Vitreomacular Traction Syndrome unmet needs.
Vitreomacular Traction Syndrome: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Herzog Carl Theodor Eye Hospital, Munich, Germany; Department of Ophthalmology, Faculty of Life Course Sciences and Medicine, King’s College London, London, UK, itreoRetinal Surgery, PA, Minneapolis, the US, among others.
Note: Detailed assessment of KOL Views will be provided in the full report on Vitreomacular Traction Syndrome.
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Vitreomacular Traction Syndrome Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Vitreomacular Traction Syndrome Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for Vitreomacular Traction Syndrome. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Vitreomacular Traction Syndrome therapies.
Vitreomacular Traction Syndrome Report Insights
- Vitreomacular Traction Syndrome Patient Population
- Therapeutic Approaches
- Vitreomacular Traction Syndrome Pipeline Analysis
- Vitreomacular Traction Syndrome Market Size and Trends
- Vitreomacular Traction Syndrome Market Opportunities
- Impact of Upcoming Therapies
Vitreomacular Traction Syndrome Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Vitreomacular Traction Syndrome Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Vitreomacular Traction Syndrome Market
- Vitreomacular Traction Syndrome Drugs Uptake
Vitreomacular Traction Syndrome Report Assessment
- Vitreomacular Traction Syndrome Current Treatment Practices
- Unmet Needs
- Vitreomacular Traction Syndrome Pipeline Product Profiles
- Vitreomacular Traction Syndrome Market Attractiveness
Vitreomacular Traction Syndrome Key Questions
- How common is Vitreomacular Traction Syndrome?
- What are the key findings of Vitreomacular Traction Syndrome epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Vitreomacular Traction Syndrome?
- What are the disease risk, burden, and unmet needs of Vitreomacular Traction Syndrome?
- At what CAGR is the Vitreomacular Traction Syndrome market and its epidemiology expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Vitreomacular Traction Syndrome market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Vitreomacular Traction Syndrome in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2020–2034)?
- How many companies are currently developing therapies for the treatment of Vitreomacular Traction Syndrome?
Ready to Dive Deeper? Purchase the Complete Report for in-depth Market Analysis by Clicking Here @ Vitreomacular traction syndrome Treatment Market Report
Access Exclusive Data Now! Click here to Read More about the Related Articles @ Latest DelveInsight Blog