Von Hippel-Lindau Disease Market
- The total Von Hippel-Lindau Market Size in the 7MM was around USD 300 million in 2023.
- WELIREG, developed by Merck, stands as the sole approved medication for Von Hippel-Lindau disease, received approval in 2021. Currently, the drug is approved for VHL associated renal cell carcinoma (RCC) and is also being studied for VHL associated pheochromocytoma/paraganglioma (PPGL) in a Phase II (NCT04924075) clinical trial.
- For Von Hippel-Lindau patients, microsurgical resection is recommended for CNS hemangioblastomas, while laser photocoagulation and cryotherapy are used for retinal capillary hemangioblastomas. Nephron-sparing surgery and percutaneous focal ablation methods are preferred for ccRCC, while radical nephrectomy is reserved for very large tumors. Laparoscopic surgical decompression and early radical resection are options for treating other Von Hippel-Lindau associated tumors such as pancreatic neuroendocrine tumors, cysts, pheochromocytoma, and endolymphatic sac tumors.
- Pipeline fro VHL is not very robust and hardly any companies have inclined their attention towards this area. Novartis is evaluating its candidate, DFF33, in early phase for treating to treat advanced/relapsed ccRCC and other malignancies with HIF2α stabilizing mutations.
- The growth of VHL disease market is expected to be mainly driven by rapid advancements in molecular diagnostics in VHL and increasing awareness of VHL disease. Several organizations such as VHL Alliance aim to create awareness and ongoing research to focus on the genetic factor of VHL disease. However, the usage of off-label medications as symptomatic treatment, limited pipeline activity and a longer duration of clinical trials expected to hit the Von Hippel-Lindau disease market growth.
Request for Sample Page of the "Von Hippel-Lindau Drugs Market"
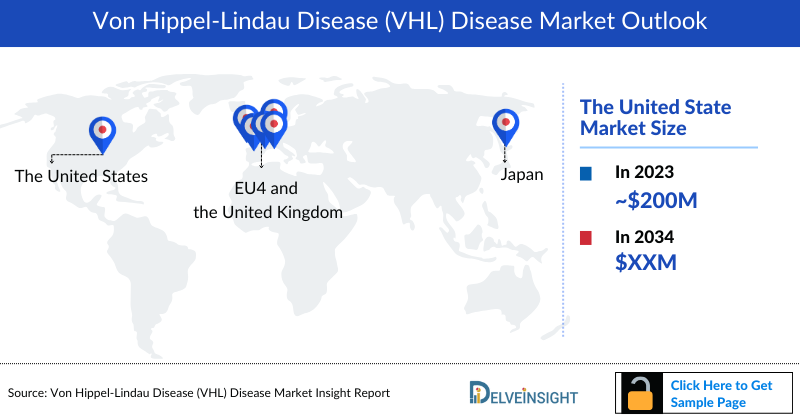
DelveInsight's “Von Hippel-Lindau Disease Disease Drugs Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of Von Hippel-Lindau disease epidemiology, market, and clinical development understanding of top oncogenic drivers/biomarkers in Von Hippel-Lindau disease , Addition to this Von Hippel-Lindau Disease Disease Drugs Market Report provides historical and forecasted epidemiology and market data as well as a detailed analysis on the Von Hippel-Lindau disease market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Von Hippel-Lindau disease Drugs Market Report provides real-world prescription pattern analysis, emerging drugs assessment, Von Hippel-Lindau Disease Disease market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted Von Hippel-Lindau disease market size from 2020 to 2034 in 7MM. The report also covers current Von Hippel-Lindau disease treatment market practices/algorithms and Von Hippel-Lindau Disease Disease unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Von Hippel-Lindau Disease Treatment Market
Von Hippel–Lindau disease is an autosomal dominantly inherited disorder that demonstrates marked phenotypic variability and age-dependent penetrance. A germline mutation in the Von Hippel-Lindau disease gene predisposes carriers to the development of abundantly vascularized tumors in multiple organs. Von Hippel-Lindau disease can be clinically classified into two types of diseases: with or without Pheochromocytoma. Those without Pheochromocytoma are categorized as Von Hippel-Lindau type 1 disease. Those with Pheochromocytoma are categorized as Von Hippel-Lindau type 2 disease. Von Hippel-Lindau type 2 disease is further classified into three categories: type 2A, type 2B, and type 2C. Type 2A Von Hippel-Lindau has Pheochromocytoma with other hemangioblastoma in the Central Nervous System (CNS) but not with Renal Cell Carcinoma. Type 2B exhibits Pheochromocytoma, Renal Cell Carcinoma, and other CNS tumors. Type 2C disease, according to recent notion, has only Pheochromocytoma, with no other disease.
Diagnostic tests include, initial screening workup including a thorough history and clinical examination, an ophthalmologic examination including fundoscopy, MR imaging of the craniospinal axis, kidneys, pancreas, and liver, an audiological examination, and laboratory tests (to detect pheochromocytomas). Genetic testing of the peripheral leukocytes and/or other body tissues is performed to confirm the presence of a Von Hippel-Lindau disease gene mutation.
The Von Hippel-Lindau disease report provide overview of Von Hippel-Lindau disease pathophysiology, diagnostic approaches and detailed treatment algorithm along with real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report
Von Hippel-Lindau Disease Treatment
Treatment for Von Hippel-Lindau disease depends on the location and size of tumors. For patients who have VHL disease and all patients who have hereditary cancer syndromes, the goal of treatment is cancer control, not to cure cancer, and preservation of functional parenchyma to avoid the morbidity associated with renal or adrenal loss. Treatment usually involves surgical removal of tumors. Radiation therapy may be used in some cases. Although there is no cure for Von Hippel-Lindau disease, the associated tumors can be treated. Early detection and treatment of tumors significantly improve a patient’s diagnosis. Left untreated, Von Hippel-Lindau disease may result in blindness, permanent brain damage, or death. Depending on the location of the tumors, the neurosurgical specialists may also collaborate with urologists, ophthalmologists, endocrinologists, and other physicians.
Von Hippel-Lindau Disease Epidemiology
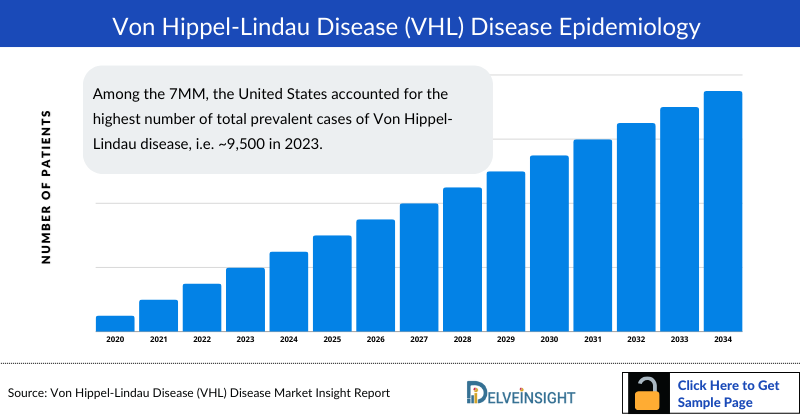
The Von Hippel-Lindau disease epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total Prevalent cases of Von Hippel-Lindau disease, total Diagnosed cases of Von Hippel-Lindau disease, Clinical Manifestation-specific cases of Von Hippel-Lindau disease, total treated cases of Von Hippel-Lindau disease in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- Among the 7MM, the United States accounted for the highest number of total prevalent cases of Von Hippel-Lindau disease, i.e. ~9,500 in 2023.
- In EU4 and the UK, Germany accounted for the highest number of total Diagnosed cases of Von Hippel-Lindau disease, whereas Spain accounts for the lowest, in 2023.
- In 2023, in the US, the most prevalent clinical manifestation-specific cases of Von Hippel-Lindau disease in the United States were attributed to retinal hemangioblastomas, making up approximately ~21% of the cases.
Total Clinical Manifestation-specific cases of Von Hippel-Lindau Disease in the United States (2023)
Unlock comprehensive insights! Click Here to Purchase the Full Report @ Von Hippel-Lindau Disease Disease Prevalence
Von Hippel-Lindau Disease Drugs Chapters
The drug chapter segment of the Von Hippel-Lindau Disease Drugs Market Report encloses a detailed analysis of Von Hippel-Lindau Disease marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Von Hippel-Lindau Disease pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Von Hippel-Lindau Disease Marketed Drugs
- WELIREG (belzutifan): Merck
WELIREG (belzutifan), developed by Merck, received FDA approval in August 2021. It's indicated for adult Von Hipple-Lindau patients with renal cell carcinoma, CNS hemangioblastomas, or pancreatic neuroendocrine tumors not immediately needing surgery. Belzutifan inhibits HIF-2α, a key factor in oxygen sensing and gene regulation under hypoxia. By binding to HIF-2α, belzutifan disrupts its interaction with HIF-1β, reducing the expression of genes linked to proliferation, angiogenesis, and tumor growth. WELIREG approval was supported by LITESPARK-004 (NCT03401788), an open-label trial involving 61 Von Hipple-Lindau disease associated RCC patients with a documented Von Hipple-Lindau disease germline alteration and at least one measurable kidney tumor according to RECIST v1.1 criteria.
Note: Detailed current therapies assessment will be provided in the full report of Von Hippel-Lindau Disease
Emerging Von Hippel-Lindau Disease Drugs
- DFF332: Novartis
DFF332 is a small molecule being developed by Novartis as a single agent and in combination in patients with advanced clear cell renal cell carcinoma and other malignancies with HIF stabilizing mutations. The development in combination includes Everolimus (RAD001, an mTOR inhibitor), and also in combination with Spartalizumab (PDR001, an anti-PD1) plus Taminadenant (NIR178, an adenosine A2A receptor antagonist). DFF332 targets a protein called HIF2α; by acting on HIF2α, DFF332 may stop the growth of certain types of cancer. DFF332 is currently being evaluated in a clinical Phase I trial to treat advanced/relapsed ccRCC and other malignancies with HIF2α stabilizing mutations.
Note: Detailed emerging therapies assessment will be provided in the final report.
Von Hippel-Lindau Disease Drug Class Insights
The existing Von Hippel-Lindau Disease treatment is mainly dominated by Hypoxia-inducible factor-2α (HIF-2α) inhibitor. Belzutifan is an inhibitor of hypoxia-inducible factor 2 alpha (HIF-2α). HIF-2α is a transcription factor that plays a role in oxygen sensing by regulating genes that promote adaptation to hypoxia. Under normal oxygen levels, HIF-2α is targeted for ubiquitin-proteasomal degradation by Von Hipple-Lindau disease protein. Lack of functional Von Hipple-Lindau disease protein results in stabilization and accumulation of HIF-2α. Upon stabilization, HIF-2α translocates into the nucleus and interacts with hypoxia-inducible factor 1 beta (HIF-1) to form a transcriptional complex that induces expression of downstream genes, including genes associated with cellular proliferation, angiogenesis, and tumor growth. Belzutifan binds to HIF-2α, and in conditions of hypoxia or impairment of Von Hipple-Lindau disease protein function, belzutifan blocks the HIF-2α-HIF-1 interaction, leading to reduced transcription and expression of HIF-2α target genes.
Von Hippel-Lindau Disease Market Outlook
For patients who have Von Hippel-Lindau disease, the goal of treatment is cancer control, not to cure cancer, and preservation of functional parenchyma to avoid the morbidity associated with renal or adrenal loss. Primary treatment of all Von Hippel-Lindau disease related tumors is local (i.e., surgical resection, RFA, radiotherapy). However, repeated local interventions at multiple sites or repeated recurrences in one area can increase morbidity. Therefore, in individual patients, systemic treatment may be an option.
Laser therapy emerges as a foremost option for patients with capillary hemangioblastomas, both with and without exudation, and may be complemented by intravitreal antiangiogenics in cases of significant exudation. Stereotactic radiotherapy presents a viable alternative for patients unsuitable for surgery or those facing challenging resections of CNS-HB. Vitreoretinal surgery becomes necessary in instances of preretinal and vitreal membrane presence, retinal detachment due to traction, and significant exudation. For hemangioblastomas near the optic nerve, intravitreal anti-VEGF therapy shows promise in halting progression, particularly for smaller lesions, and may alleviate exudates and edema in select cases. Salvage external beam radiation serves as an option for treatment-resistant retinal hemangioblastomas, offering tumor volume reduction and improved visual acuity. Bevacizumab, a humanized monoclonal antibody targeting soluble VEGF, has shown efficacy in patients with HB and RCH. Additionally, salvage treatments targeting VEGF, platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) are available for localized tumors not amenable to local procedures.
Key Von Hippel-Lindau Disease Companies, such as Merck, Novartis and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Von Hippel-Lindau disease.
- Among the 7MM, the United States accounted for the highest Von Hippel-Lindau Disease Market Size in with nearly USD 200 million, in 2023.
- Currently, WELIREG (belzutifan) dominates the Von Hippel-Lindau disease market.
- However, the development of new emerging drugs seems to be slow and lacking, due to which only a few emerging drugs can be seen in the pipeline. Also due to expected less competition and rarity of disorder, companies are expected to charge the premium pricing for therapies on approval and the healthcare payers might restrict the usage of the drug in the broader VHL population.
Von Hippel-Lindau Disease Drugs Uptake
This section focuses on the uptake rate of potential Von Hippel-Lindau Disease drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report.
Von Hippel-Lindau Disease Activities
The Von Hippel-Lindau Disease therapeutics market report provides insights into different therapeutic candidates in Phase I and Phase II stages. It also analyzes key Von Hippel-Lindau Disease Companies involved in developing targeted therapeutics.
Von Hippel-Lindau Disease Pipeline Development Activities
The Von Hippel-Lindau Disease theraeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Von Hippel-Lindau disease emerging therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Von Hippel-Lindau Disease Treatment Drugs
KOL Views
To keep up with the real world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Medical Oncologists, Pulmonologists and Professors, Chief of the Thoracic Service at the Memorial Sloan Kettering Cancer Center, and Others.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as MD Anderson Cancer Center, Texas, UT Southwestern Medical Center in Dallas, Cancer Research UK Barts Centre in London, LUNGevity Foundation, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Von Hippel-Lindau disease market trends.
Von Hippel-Lindau Disease Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Von Hippel-Lindau Disease Drugs Market Access and Reimbursement
Reimbursement of rare disease therapies can be limited due to lack of supporting policies and funding, challenges of high prices, lack of specific approaches to evaluating rare disease drugs given limited evidence, and payers’ concerns about budget impact. The high cost of rare disease drugs usually has a limited effect on the budget due to the small number of eligible patients being prescribed the drug.
Bevacizumab was assessed by the National Institute for Health and Care Excellence (NICE) in England. The manufacturer had submitted a dossier that included a multicentre, randomised, double-blind phase III trial comparing the combination of bevacizumab and interferon alfa-2a with interferon alfa-2a plus placebo in support of its application. However, on the basis of unproven cost-effectiveness of the treatment, NICE gave a negative reimbursement decision.
The Von Hippel-Lindau Disease Treatment Market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Von Hippel-Lindau Disease Therapeutics Market Report Scope
- The Von Hippel-Lindau Disease therapeutics market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression along with treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current Von Hippel-Lindau Disease Treatment Market landscape.
- A detailed review of the Von Hippel-Lindau Disease Therapeutics Market, historical and forecasted Von Hippel-Lindau Disease market size, Von Hippel-Lindau Disease market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Von Hippel-Lindau Disease Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Von Hippel-Lindau Disease Therapeutics Market.
Von Hippel-Lindau Disease Therapeutics Market Report Insights
- Patient-based Von Hippel-Lindau Disease Market Forecasting
- Therapeutic Approaches
- Von Hippel-Lindau Disease Pipeline Analysis
- Von Hippel-Lindau Disease Market Size
- Von Hippel-Lindau Disease Market Trends
- Existing and future Von Hippel-Lindau Disease Therapeutics Market Opportunity
Von Hippel-Lindau Disease Therapeutics Market Report Key Strengths
- 11Years Von Hippel-Lindau Disease Market Forecast
- 7MM Coverage
- Von Hippel-Lindau Disease Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Von Hippel-Lindau Disease Drugs Uptake
- Key Von Hippel-Lindau Disease Market Forecast Assumptions
Von Hippel-Lindau Disease Treatment Market Report Assessment
- Current Von Hippel-Lindau Disease Treatment Market Practices
- Von Hippel-Lindau Disease Unmet Needs
- Von Hippel-Lindau Disease Pipeline Product Profiles
- Von Hippel-Lindau Disease Treatment Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the historical and forecasted Von Hippel-Lindau Disease patient pool/patient burden in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What was the Von Hippel-Lindau Disease market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Which combination treatment approaches will have a significant impact on Von Hippel Lindau disease treatment market size?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of Von Hippel-Lindau Disease?
- How many companies are developing therapies for the treatment of Von Hippel-Lindau Disease?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy
- The Von Hippel-Lindau Disease Treatment Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Von Hippel-Lindau Disease Treatment Market.
- Insights on patient burden/disease Von Hippel-Lindau Disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Patient-based forecast model which uses bottom-up forecasting techniques is accepted as a gold standard in pharma forecasting.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the Von Hippel-Lindau Disease unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.
Read More about the Related Articles:-