WRN Inhibitors Market Summary
- WRN inhibitors target the Werner helicase, a key protein in DNA repair and genome stability, offering a highly selective approach for cells with microsatellite instability (MSI).
- Secondary data indicate that 40–70% of patients with MSI-positive cancers either do not respond or develop resistance to immunotherapy, highlighting the critical need for alternative treatment strategies. WRN helicase, a central enzyme in DNA repair, has therefore emerged as a compelling therapeutic target in these tumors.
- The WRN inhibitors landscape is still in its early stages, with no approved therapies, offering a compelling opportunity for pioneering research, innovation, and clinical development. The scarcity of targeted options for MSI-high, high-risk tumors further underscores the strategic significance and potential transformative impact of WRN-focused research within the precision oncology field.
- Preclinical studies demonstrate that WRN inhibition induces synthetic lethality in MSI-high cells, highlighting a strong biological and mechanistic foundation for clinical investigation.
- NDI-219216 demonstrated superior activity compared with other clinical-stage WRN inhibitors (Novartis HRO761, Roche RO7589831) across multiple MSI-H preclinical models.
- In June 2025, Bayer’s Vividion Therapeutics secured the global rights from its partner Roche to the only covalent WRN inhibitor to have made it into the clinic. Vividion and Roche have collaborated on the candidate, known as RO7589831, as part of their 2020 partnership. The drug is designed to damage the DNA of cancers by inhibiting WRN, a DNA repair enzyme.
- In July 2025, MOMA Therapeutics announced that the first patient had been dosed in its Phase I clinical trial to assess the safety and tolerability of MOMA-341.
- The concentration of early-stage research allows for first-mover advantage, combination strategy development, and expansion into broader DNA repair–deficient oncology indications.
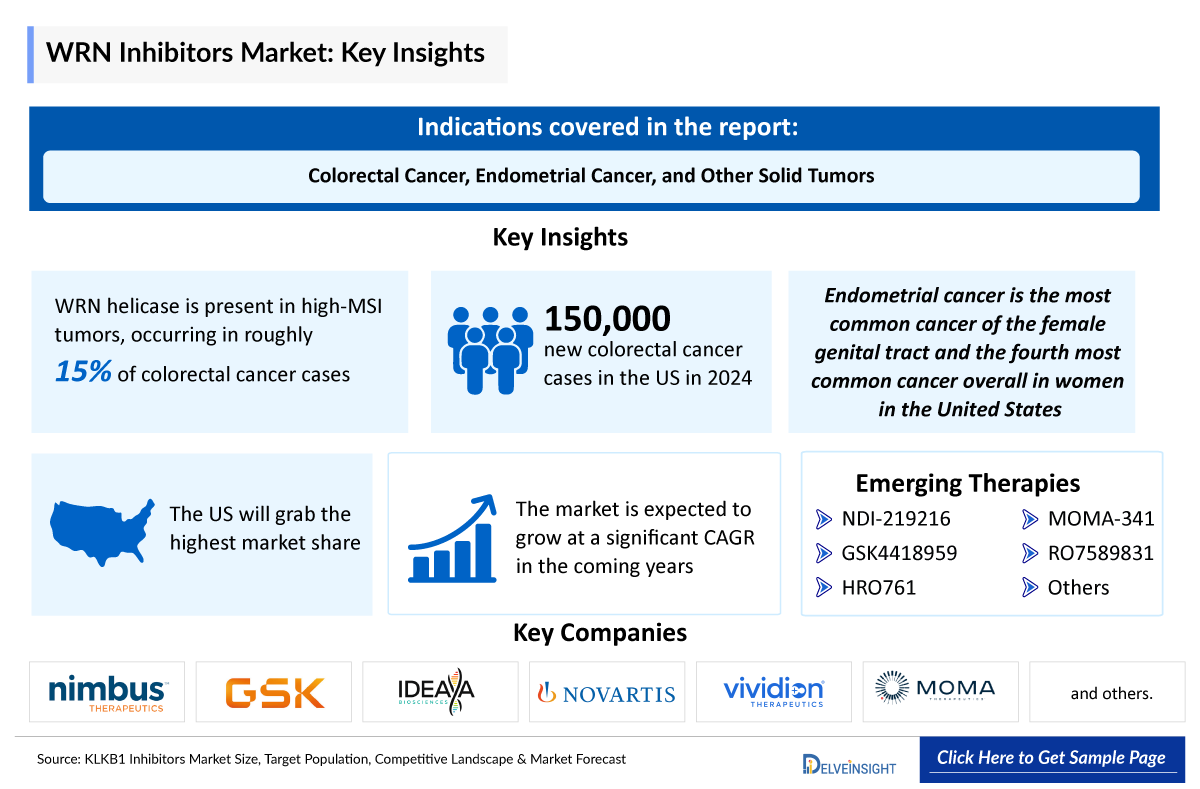
- Several early-stage WRN inhibitors are under development by leading biotech and pharma companies, including NDI‑219216 (Nimbus Therapeutics), GSK4418959 (GlaxoSmithKline and Ideaya Biosciences), HRO761 (Novartis Pharmaceuticals), and VVD-214 (RO7589831) (Vividion Therapeutics), highlighting growing scientific and commercial interest in this mechanism.
DelveInsight’s “WRN Inhibitors – Target Population, Competitive Landscape, and Market Forecast – 2040” report delivers an in-depth understanding of the WRN inhibitors, historical and competitive landscape, as well as the WRN inhibitors market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The WRN inhibitors market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM WRN inhibitors market size from 2020 to 2040. The report also covers WRN inhibitors treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2040
|
Study Period |
2020–2040 |
|
Forecast Period |
2025–2040 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan |
|
WRN Inhibitors Epidemiology |
Segmented by:
|
|
WRN Inhibitors Key Companies |
|
|
WRN Inhibitors Key Therapies |
|
|
WRN Inhibitors Market |
Segmented by:
|
|
Analysis |
|
WRN Inhibitors Disease Understanding and Treatment Algorithm
WRN Inhibitors Overview
Werner helicase (WRN) inhibitors are an emerging area of research targeting the WRN protein, a critical protein involved in DNA repair and maintaining genome stability. These inhibitors are particularly relevant in cells with microsatellite instability (MSI), where WRN function is essential for preserving genomic integrity. Although no WRN-targeting agents have yet been approved, the mechanism has garnered significant scientific interest due to its potential implications in understanding DNA damage response, cellular proliferation, and genomic maintenance.
Further details related to country-based variations are provided in the report.
WRN Inhibitors Epidemiology
The WRN Inhibitors epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total cases of selected indications for WRN Inhibitors, total eligible patient pool of selected indications, total treated cases in selected indications for WRN Inhibitors in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2040.
- WRN helicase is present in high-MSI tumors, occurring in roughly 15% of colorectal cancer cases.
- Colorectal cancer is more commonly observed in men than in women, which may be attributed to higher exposure to risk factors such as smoking, alcohol consumption, and dietary habits.
- In 2024, the US recorded approximately 150,000 new colorectal cancer cases, the highest among the 7MM, likely due to lifestyle factors, dietary patterns, and extensive screening programs that increase detection.
- The disease is most frequently diagnosed in individuals aged 45 to 84 years, reflecting the cumulative effects of aging, long-term exposure to risk factors, and age-related changes in cellular repair mechanisms.
- Endometrial cancer is the most common cancer of the female genital tract and the fourth most common cancer overall in women in the United States, with approximately 61,000 newly diagnosed cases annually.
- Endometrial cancer has a peak incidence in those between 65 and 75 years. About 69% of uterine cancers are diagnosed when the cancer is in an early stage. This is largely because abnormal vaginal bleeding is an early symptom, particularly when it occurs after menopause.

WRN Inhibitors Drug Chapters
The drug chapter segment of the WRN report encloses a detailed analysis of mid and early-stage (Phase I/II, and Phase I) pipeline drugs. It also helps to understand the WRN’s clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Emerging Drugs
NDI-219216: Nimbus Therapeutics
NDI-219216 is a highly potent and selective non-covalent investigational inhibitor of Werner syndrome helicase activity being developed for the treatment of microsatellite instability-high (MSI-H) tumors. The company is currently conducting a multicenter, open-label Phase I/II trial (NCT06898450) with NDI-219216 in patients with advanced solid tumors with/without MSI.
In April 2025, Nimbus Therapeutics announced that the Phase I/II clinical trial of NDI-219216 is actively enrolling and dosing patients with advanced solid tumors. Nimbus presented promising preclinical data on NDI-219216 (previously NTX-452) at the 36th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics in October 2024.
GSK4418959: GlaxoSmithKline and IDEAYA Biosciences
IDE275's (GSK959) potential as a best-in-class WRN inhibitor, inducing single-agent tumor regressions in MSI-H patient-derived xenograft (PDX) and cell line-derived xenograft (CDX) models for endometrial, colorectal, and gastric cancers. It is currently in Phase I/II of development for the treatment of adult participants with mismatch repair-deficient (dMMR) or MSI-H solid tumors, including endometrial, colorectal, and others.
- In March 2025, IDEAYA Biosciences announced the publication of abstracts for an oral presentation in the New Drugs on the Horizon series, and three poster presentations on IDE275 (GSK959) at the AACR Annual Meeting 2025. IDE275 has demonstrated selective preclinical efficacy in the high microsatellite instability (MSI-H) solid tumor setting and is advancing in a Phase I dose escalation trial in partnership with GSK.
- In June 2020, IDEAYA Biosciences and GlaxoSmithKline announced a strategic partnership in Synthetic Lethality, an emerging field in Oncology. The strategic partnership includes IDEAYA’s Synthetic Lethality programs MAT2A, Pol Theta, and Werner Helicase programs, which are projected to reach clinical trials within the next three years.
|
Emerging Drugs | |||||
|
Product |
Company |
RoA |
Phase |
WRN Inhibitors types (Covalent/Non-covalent) |
Indication |
|
NDI-219216 |
Nimbus Therapeutics |
Oral |
I/II |
Non-covalent |
Advanced solid tumors with/without MSI. |
|
GSK4418959 |
GlaxoSmithKline and Ideaya Biosciences |
Oral |
I/II |
Non-covalent |
Adult participants with dMMR or MSI-H solid tumors, including those for endometrial, colorectal, and others. |
|
HRO761 |
Novartis Pharmaceuticals |
Oral |
I |
Non-covalent |
Patients with dMMR or MSI-H solid tumors, including colorectal cancers. |
|
VVD-214 (RO7589831) |
Vividion Therapeutics |
Oral |
I |
Covalent |
Advanced solid tumors with dMMR or MSI. |
|
MOMA-341 |
MOMA Therapeutics |
Oral |
I |
Covalent |
Metastatic colorectal cancer with high MSI-H. |
Note: Detailed emerging therapies assessment will be provided in the final report.
WRN Inhibitors Market Outlook
WRN inhibitors remain in the early development stage, with no therapies yet approved by regulators. Interest in this mechanism is growing due to the critical role of WRN in DNA repair and genome stability, particularly in tumors with MSI, positioning WRN inhibition as a promising strategy for precision oncology.
Emerging candidates such as NDI‑219216, GSK4418959, HRO761, and RO7589831 are currently in early clinical development, highlighting both scientific validation and commercial potential. These agents aim to exploit synthetic lethality in MSI-high tumors, offering a targeted approach for cancers that currently have limited therapeutic options. The potential for combination strategies and expansion into other DNA repair–deficient cancers represents a significant opportunity for innovation.
Overall, WRN inhibitors represent an emerging therapeutic class with transformative potential in precision oncology. With promising early-phase candidates, a strong mechanistic rationale, and increasing scientific interest, this space is poised for long-term growth as development progresses from preclinical studies toward clinical validation.
WRN Inhibitors Drugs Uptake
This section focuses on the uptake rate of potential emerging WRN inhibitors expected to be launched in the market during 2025–2040.
WRN Inhibitors Pipeline Development Activities
The report provides insights into different therapeutic candidates in the early stages. It also analyzes key players involved in developing targeted therapeutics.
The presence of numerous drugs at different stages is expected to generate immense opportunities for the WRN inhibitors market growth over the forecasted period.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for WRN inhibitor therapies.
KOL Views
To keep up with current and future market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on WRN inhibitors’ evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as Johns Hopkins Sidney Kimmel Cancer Center and others.
Their opinion helps understand and validate current and emerging therapy treatment patterns or WRN inhibitors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
KOL Views |
|
“The Werner syndrome RecQ helicase WRN was identified as a synthetic lethal target in cancer cells with microsatellite instability (MSI) by several genetic screens. Despite advances in treatment with immune checkpoint inhibitors, there is an unmet need in the treatment of MSI cancers.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of the WRN inhibitors, explaining its mechanism and emerging therapies.
- Comprehensive insight into the Competitive Landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of emerging therapies and the elaborate profiles of early-stage and prominent therapies will impact the current landscape.
- A detailed review of the WRN inhibitor market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM WRN inhibitor market.
WRN Inhibitors Report Insights
- WRN Inhibitor Targeted Patient Pool
- Therapeutic Approaches
- WRN Inhibitor Pipeline Analysis
- WRN Inhibitor Market Size and Trends
- Existing and future Market Opportunity
WRN Inhibitor Report Key Strengths
- Fifteen years Forecast
- The 7MM Coverage
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
WRN Inhibitor Report Assessment
- Current Treatment Practices
- Unmet Needs
- Analyst Views
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT Analysis)
Key Questions
- What was the WRN inhibitor total market size, the market size by therapies, market share (%) distribution, and what would it look like in 2040? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2040?
- What are the risks, burdens, and unmet needs of treatment with WRN inhibitors? What will be the growth opportunities across the 7MM for the patient population of WRN inhibitors?
- What are the key factors hampering the growth of the WRN inhibitor market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What regulatory designations have been granted for WRN-targeted therapies?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
Reasons to buy
- The report will help develop business strategies by understanding the latest trends and changing dynamics driving the WRN inhibitor Market.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights into the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.