
Novel biomarkers: Rheumatic & Musculoskeletal Diseases
- Home
- Eular Conference
- novel biomarkers in rheumatic diseases
Rheumatic and Musculoskeletal Diseases Highlights
Introduction
Rheumatic diseases, also called musculoskeletal diseases, are characterized by pain and a consequent reduction in the range of motion and function in one or more areas of the musculoskeletal system; in some diseases, there are signs of inflammation: swelling, redness, warmth in the affected areas. These diseases also tend to affect internal organs. Rheumatic and musculoskeletal diseases (RMDs) are chronic systemic diseases that commonly affect the joints but can affect any body organ. There are more than 200 RMDs that affect children and adults. It has been estimated that approximately 46 million people in the United States live with some rheumatic disease, which has been the most common cause of reduced mobility.
Although rheumatic conditions are the most common medical problems, many individuals’ diseases are uncommon. Several factors have been considered the prominent factors for inclusion in the group's Rheumatic and Musculoskeletal Diseases (RMDs). First, it should be emphasized that RMDs encompass many different diseases which could affect persons at any age. Second, it needs to be clear that there are different pathological causes of RMDs. In addition, the impact of RMDs on individuals and society should be emphasized. The most prevalent and well-known examples of RMDs are Rheumatoid Arthritis (RA), Osteoarthritis (OA), and Gout.
As per the conservative estimate of the United Nations, symptomatic OA, or degenerative joint disease, affects 15% of people worldwide, and it is estimated that by 2050, over 130 million people will suffer from OA worldwide, and 40 million will be severely disabled. Rheumatoid Arthritis (RA) is the most common form of autoimmune inflammatory form of arthritis, and it affects nearly 1 in 100 persons worldwide. Gout is another form of inflammatory arthritis that most commonly affects men and has a prevalence of 4% in the US and Europe. Several other distinct forms of RMDs are known, which are less common but cause significant morbidity and mortality (van der Heijde et al., 2018).
Rising Disease Burden of Musculoskeletal Disease
According to World Health Organization (WHO), a recent analysis of Global Burden of Disease (GBD) data showed that approximately 1.71 billion people globally have musculoskeletal conditions. People of all the ages everywhere around the world are getting affected due to the rising prevalence of musculoskeletal conditions, but the countries with the high income are the most affected in terms of the number of people – 441 million, followed by countries in the WHO Western Pacific Region with 427 million and South-East (World Health Organization, 2021).
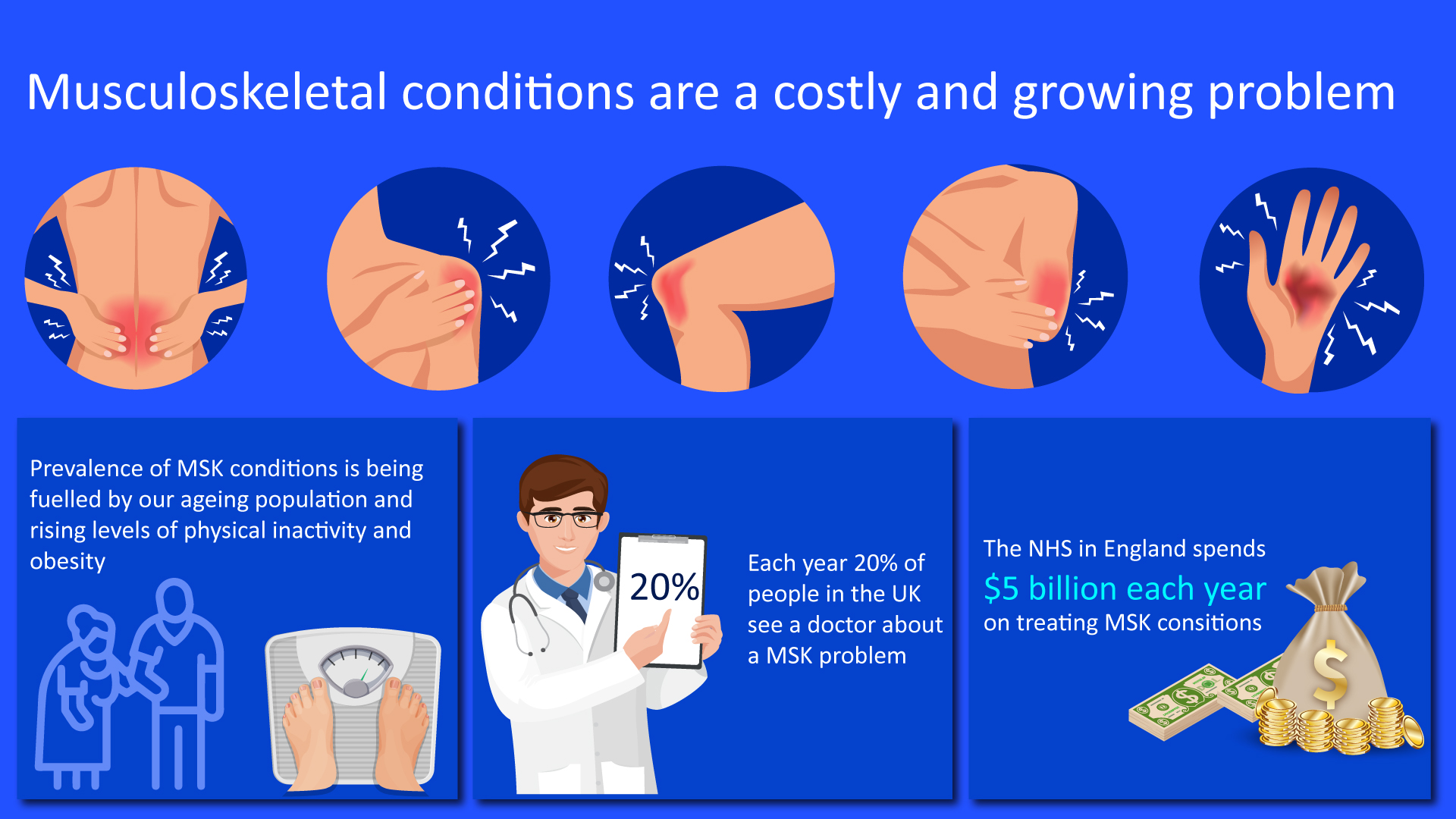
Figure 1: Cost associated with the treatment of musculoskeletal diseases
According to UK Health Security Agency, Arthritis and musculoskeletal (MSK) conditions affect over 17 million people across the UK, causing pain, disability, fatigue, and often anxiety, depression, or social isolation. Major symptoms associated with RMDs include pain and joint stiffness, limited movement, making activities such as spending time with family and friends, or looking after a household much harder. Over half of people (57%) living with arthritis say they experience pain every day. These conditions disproportionately affect those who are socially and economically deprived. The impact of arthritis also stretches beyond the individual to services and the economy (UK Health Security Agency, 2019).
Psychosocial aspects of rheumatic and musculoskeletal diseases
The majority of the population with rheumatic and musculoskeletal diseases experience a sudden change from the moment the patient receives a diagnosis in the cognitive, emotional, and behavioral levels. A Series of clinical symptoms begin, which may affect the patient’s mobility to different degrees and also lead to diminishing or loss of some daily life functions; this leads to the direct or indirect alterations to the person’s financial independence.
There is a need to consider this disease from a biopsychosocial perspective as a system where modifying one dimension may lead to change in others. In this manner, the severity of the disease may lead to an increase in negative emotions (anxiety, rage, or sadness), pain, disability, and reduction of the ability to work and carry out everyday tasks and social activities. The currently recommended biopsychosocial model for an approach to any pathology calls upon the detection and treatment of patients’ psychological and social problems, not a mere focus on their physical problems since it has been proven that control of psychological problems, offering information and correct adaptation improves the doctor–patient relationship and the course of the disease.
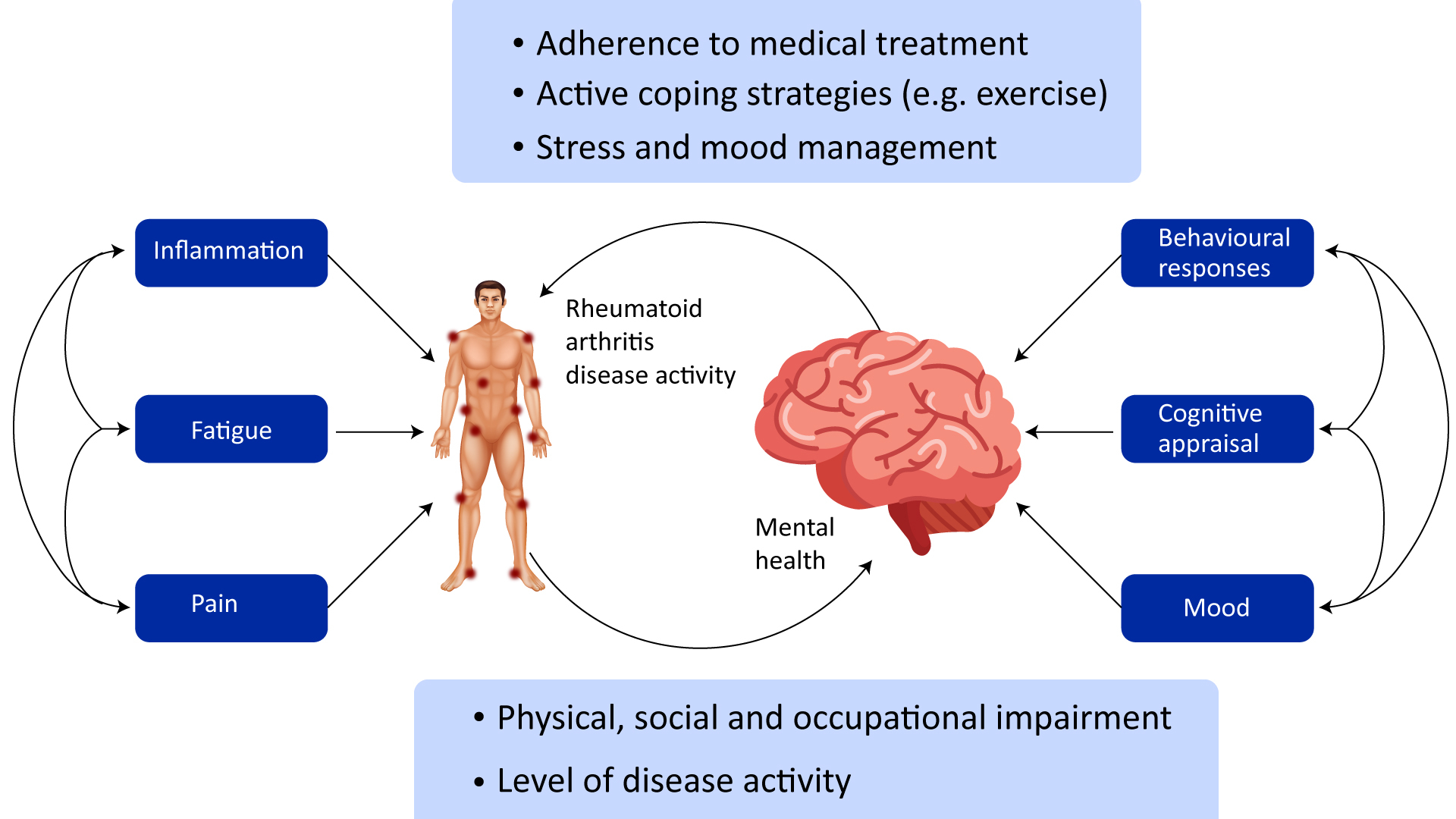
Figure 2: Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways
Individuals with rheumatoid arthritis (RA) demonstrate an increased prevalence of mood disorders compared with the general population but a decreased prevalence of schizophrenia, probably due to alterations in inflammatory processes. Several mechanisms underlie the relationship between RA and mental health comorbidities: cognitive, behavioral, and affective responses, inflammatory processes, and fatigue (Sturgeon et al., 2016).
Biomarkers in Rheumatology
The development of robust biomarkers in rheumatology will lay the foundation for precision medicine and aid in identifying the disease risk and improving diagnosis and prognosis. Even though some biomarkers are routinely used to diagnose and treat rheumatic disease, there is an unmet need for novel biomarkers in clinical practice and drug development. Therefore, progress needs to be made in biomarker discovery and development for rheumatic diseases. The advancements in the next-generation technologies, including large-scale sequencing, proteomic technologies, metabolomics technologies, mass cytometry, and multianalyte analysis technologies, have resulted in potential new biomarkers.
Need for Biomarkers
Currently, the etiology of RA remains unknown; multiple mechanisms are thought to be involved in the phytopathogenic chain. The progress made in the pathogenesis of RA processes has led to the interest in studying the biomarkers involved in different stages of the disease.

The use of biomarkers in rheumatology can help identify disease risk, improve diagnosis and prognosis, target therapy, assess response to treatment, and further our understanding of the underlying pathogenesis of the disease (Robinson & Mao, 2016).
Biomarkers to guide clinical therapeutics in rheumatology
Biological markers are widely used in medicine and can objectively measure normal and pathogenic processes or pharmacological responses to a therapeutic intervention. Many obstacles impede the biomarker development process. Although rarely discussed, the reality is that there is an abundance of candidate biomarkers available, but very few have undergone extensive validation. The biomarkers currently used in the practice of rheumatology can be subdivided into three distinct molecular classes:
Molecular biomarkers
Molecular biomarkers are biochemical variables such as measurements of nucleic acids, proteins, lipids, carbohydrates, metabolites, and other biomolecules in the blood, synovial fluid and other bodily fluids, and tissues. Objective, quantitative measurements of molecular biomarkers through various techniques serve as indicators of normal or pathologic processes or responses to therapy. The advent of new technologies such as large-scale nucleic acid sequencing, proteomics, lipidomics, glycomics, metabolomics, and mass cytometry has enabled the next-generation molecular biomarkers identification.
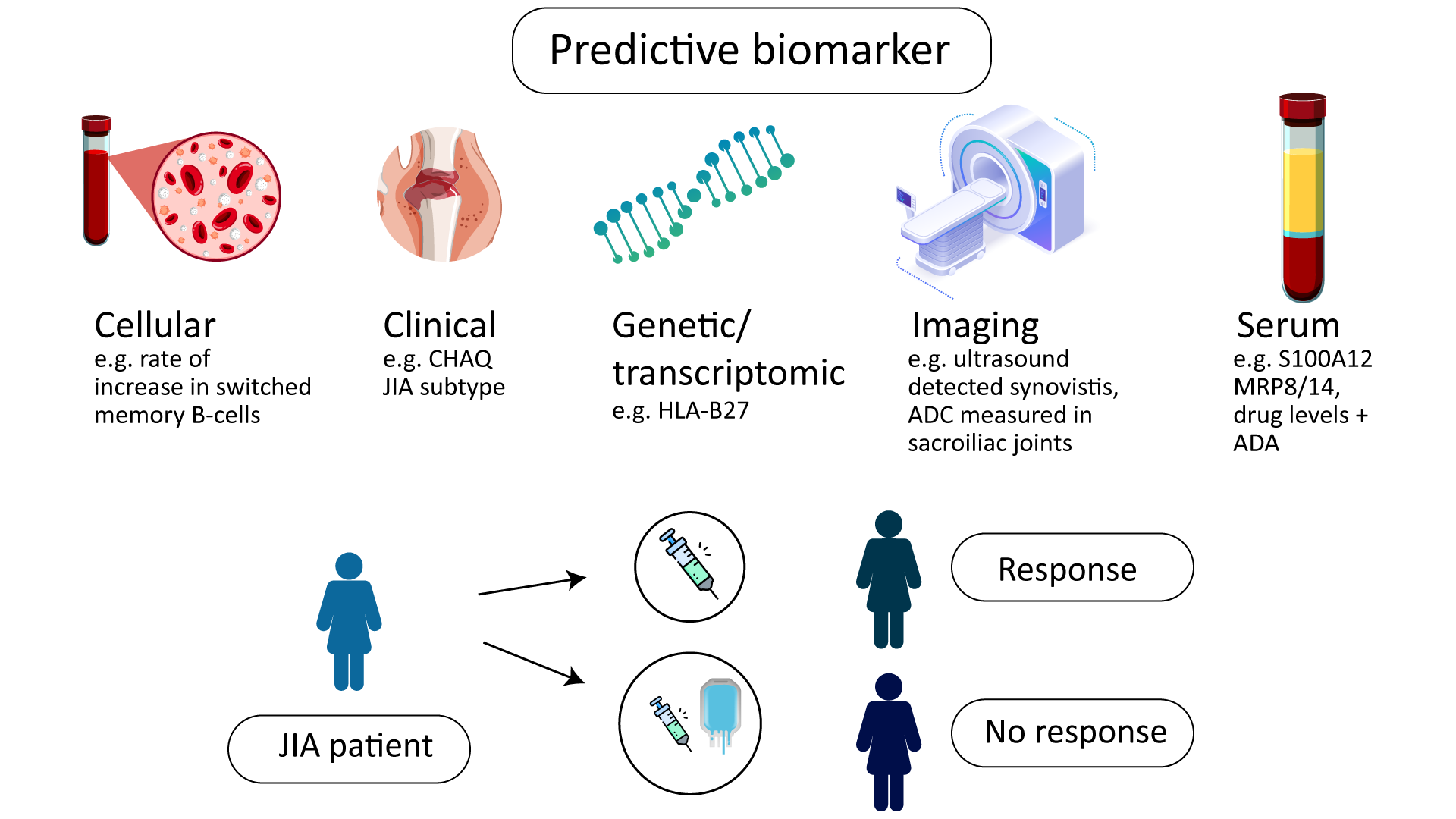
Figure 3: Biomarkers of Response to Biologic Therapy in Juvenile Idiopathic Arthritis
Imaging biomarkers
Imaging technologies such as MRI, PET-computed tomography, and ultrasound provide biomarkers that enable the assessment of disease activity and response to treatments by visualizing anatomical and structural changes. Imaging methods are generally noninvasive, preventing the need for collecting samples from patients. Compared with molecular biomarkers, imaging biomarkers have frequently been more closely associated with the phenotypic manifestations of established diseases. Moreover, imaging allows structural and functional evaluations of disease activity and therapy.
Clinical biomarkers
Clinical biomarkers are typically physical variables or symptoms, such as joint counts (i.e., the number of swollen and tender joints), pain scores, level of proteinuria, and other clinical findings. Although contributing to the diagnosis and assessment of established disease, clinical biomarkers have generally not provided value in guiding the selection of therapies for treating patients with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), or vasculitis.
Application of Biomarkers in Rheumatology
Diagnostic biomarker: used to detect or confirm the presence of a disease or certain condition or to identify individuals with a subtype of the disease. For example, HbA1c is commonly the most used biomarker to diagnose prediabetes and diabetes.
Prognostic biomarker: used to identify the probability of a clinical event, disease recurrence, or progression in patients diagnosed with a disease or medical condition of interest. For example, increasing prostate-specific antigen (PSA) is a predictor of the clinical progression of prostate cancer.
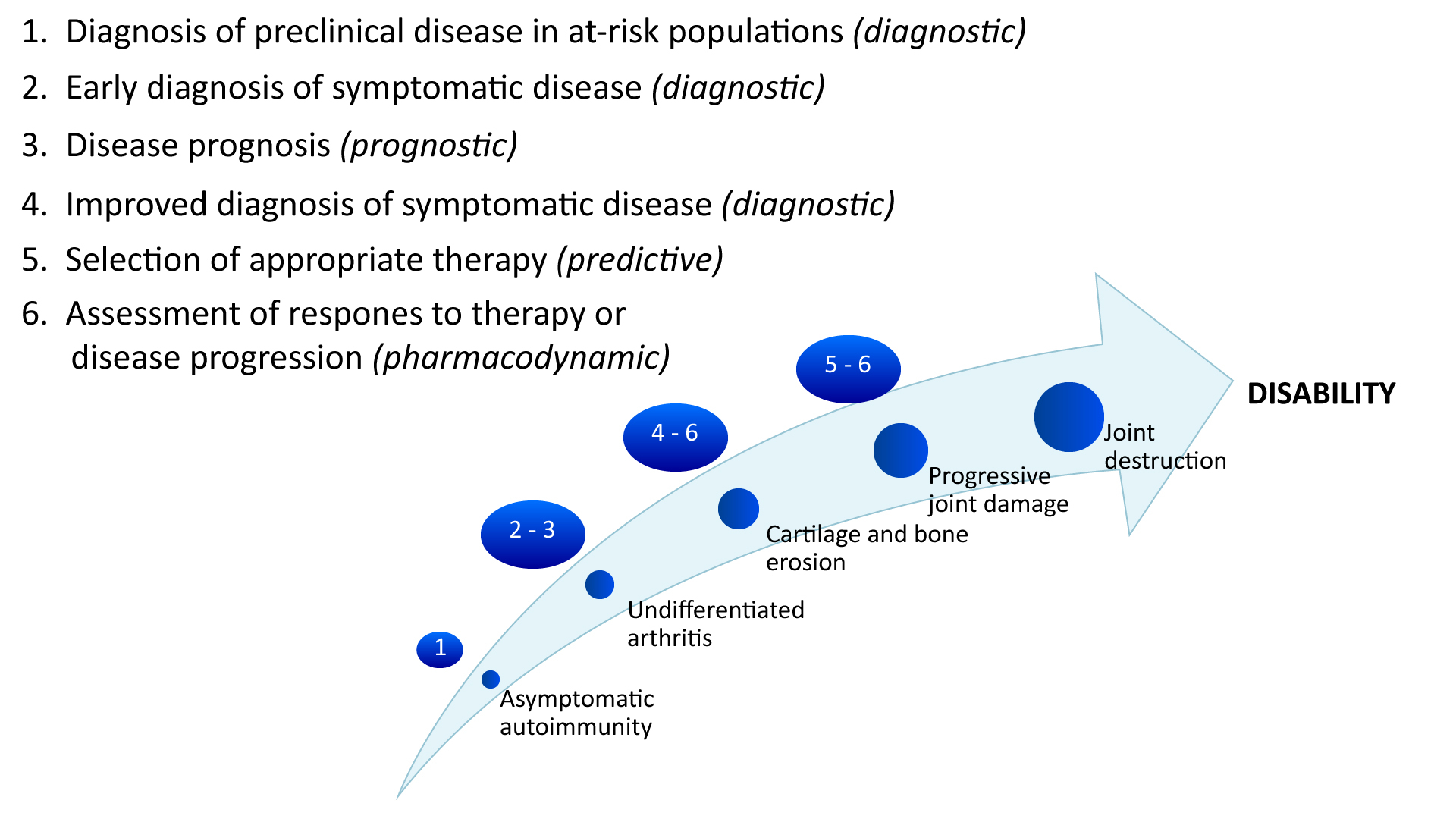
Figure 4: Applications of biomarkers at different stages in the development of rheumatic diseases.
Safety biomarker: used to indicate the likelihood, presence, or extent of toxicity as an adverse effect measured before or after exposure to a medical product or derived from environmental causes. For example, transaminases have been selected as biomarkers for potentially hepatotoxic drugs.
Monitoring biomarker: measured in series to assess the level of a disease or medical condition or the evidence of exposure to (or the effect of) a medical product or environmental agent. For example, B-type natriuretic peptide as a measure of vascular and ventricular function in pediatric pulmonary arterial hypertension.
Pharmacodynamic response biomarker: used to demonstrate that in exposition to medical products or environmental causes in an individual, there is a biological reaction. For example, International Standardized Ratio (INR) for anticoagulant treatment has a special interest in the adjustment of the drug (Puentes-Osorio et al., 2021).
Biomarkers in Rheumatoid Arthritis
Inflammatory rheumatic diseases (IRD) constitute a wide spectrum of disorders encompassing inflammatory arthropathies, such as rheumatoid arthritis (RA), axial spondyloarthritis (ax-SpA), and psoriatic arthritis (PsA). It also includes connective tissue disorders, like systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), or systemic sclerosis (SSc). This highly heterogeneous group of conditions has multifactorial and not fully understood etiology. It is primarily characterized by persistent inflammation affecting the musculoskeletal system and connective tissue. Disease progression ultimately leads to organ damage, functional disability, premature mortality, as well as economic and social burdens. Rheumatoid arthritis and SpA represent the most common IRD (Saas et al., 2022). However, existing biomarkers have limitations concerning RA diagnosis. New protein biomarkers potentially detected through serum protein profiling are expected to represent various physiological changes in RA, besides inflammation and immunity (Mun et al., 2021).
Autoantibodies against Citrullinated Proteins (ACPA)
Autoantibodies to citrullinated protein epitopes have been a focus of biomarker research in RA for many years. Citrullination is a post-translational modification of proteins that can generate new epitopes to which the immune system is not tolerant, leading to new autoantibodies. Amongst ACPAs, the assay for anti-cyclic citrullinated peptide (anti-CCP2) is widely clinically available and has excellent diagnostic and prognostic value. Both RF and anti-CCP2 have similar sensitivities for the diagnosis of RA, but anti-CCP2 is more specific.
Erythrocyte Sedimentation Rate (ESR) and C-reactive Protein (CRP)
The ESR, the rate at which erythrocytes fall through plasma when suspended in a vertical tube, is an indirect measure of the levels of acute-phase reactants (mainly fibrinogen). ESR levels are influenced by several factors, such as the size, shape, and number of red blood cells and other plasma constituents like immunoglobulins. Elevated ESR levels may be caused by systemic or local inflammatory processes, infection, malignancy, tissue injury, end-stage renal disease, nephrotic syndrome, and obesity. CRP is an acute-phase reactant in the pentraxin protein family, which comprises pattern recognition molecules involved in the innate immune response. CRP occurs in both acute and chronic inflammatory states, infectious and noninfectious. Low-grade CRP elevation is associated with various metabolic stressors, including but not limited to atherosclerosis, obesity, type 2 diabetes, sedentary lifestyles, and an unhealthy diet. Furthermore, there is no standardized reference range or unit of measure for CRP values; these vary between laboratories
Although ESR and CRP measurements are imperfect, both continue to play a role in diagnosing and managing RA. Elevated ESR and CRP levels are included in the 2010 ACR/EULAR Classification Criteria for RA.
|
Table 1: Potential predictive biomarkers for rheumatoid arthritis treatment. | ||
|
Biomarkers |
Presence/Absence |
Medication/Drugs |
|
Anti-CCP |
Present |
Rituximab |
|
Anti-MCV |
Present |
Rituximab |
|
14-3-3 eta |
Absent/Low level |
Tocilizumab |
|
Cartilage Oligometic Matrix Protein (COMP) |
Absent/Low level |
Adalimumab |
|
Calprotectin |
Present |
Adalimumab, Infliximab, Rituximab |
|
Survivin |
Absent/Low level |
Infliximab |
Fibrinogen-like Protein 1
FGL1, called hepatocyte derived fibrinogen-related protein 1 or hepassocin, belongs to the fibrinogen family, with a high degree of amino acid homology with the carboxyl terminus of the fibrinogen β- and γ-subunits, but it does not have a platelet-binding site, cross-linking region, or thrombin-sensitive site, which are necessary for fibrin clot formation. The association of upregulated circulating FGL1 with disease activity is beneficial for inhibiting autoimmunity but enhances the risks of obesity, diabetes, and cardiovascular events. Finally, the FGL1-LAG-3 pathway could become a novel intervention target for RA treatment (Liu et al., 2020).
Multi-biomarker Disease Activity (MBDA) Test
The MBDA test is a commercially available assay that measures 12 serum protein biomarkers and applies an algorithm to summarize the information into a single score that indicates the level of “RA inflammation.” The following biomarkers are tested: vascular cell adhesion molecule-1 (VCAM-1), epidermal growth factor (EGF), vascular endothelial growth factor A (VEGF-A), interleukin 6 (IL-6), tumor necrosis factor receptor type 1 (TNF-R1), matrix metalloproteinase-1 (MMP-1), matrix metalloproteinase-3 (MMP-3), human cartilage glycoprotein 39 (YKL-40), leptin, resistin, serum amyloid (SAA), and CRP. The cost-effectiveness and role of this test in routine clinical practice remain controversial.
Biomarkers in Osteoarthritis
Biomarkers, especially biochemical markers, are important in osteoarthritis (OA) research, clinical trials, and drug development and have the potential for more extensive use in therapeutic monitoring. However, they have not yet had any significant impact on disease diagnosis and follow-up in a clinical context. Nevertheless, the development of immunoassays for detecting and measuring biochemical markers in OA research and therapy is an active area of research and development. The evaluation of biochemical markers representing low-grade inflammation or extracellular matrix turnover may permit OA prognosis and expedite the development of personalized treatment tailored to fit particular disease severities. However, detection methods have failed to overcome specific hurdles such as low biochemical marker concentrations, patient-specific variation, and limited utility of single biochemical markers for definitive characterization of disease status. These challenges require new and innovative approaches for developing detection and quantification systems that incorporate clinically relevant biochemical marker panels.
The biomarker for OA should reflect dynamic and quantitative changes in joint tissues. There is accumulating evidence that these biomarkers can distinguish the cartilage breakdown changes in the cartilage of knee OA and diagnose functional, structural, and biochemical changes and associated damage in the bone and the subchondral tissue. Several biomarkers have been proposed in OA over the years for an early-stage diagnosis, monitoring disease progression or severity, and examining drug potential’s efficacy. The identified biomarkers should be compatible with pain score, clinical and radiological findings of the disease, and the smallest changes in concentration of biomarkers should be related to disease severity and pathology. These biomarkers include proteins, metabolites, carbohydrate biomarkers, genomic biomarkers, cellular biomarkers, and imaging biomarkers and are usually measured in a selected body fluid such as blood, serum, urine, synovial fluid, and cartilage tissue. These markers are reflected in bone loss, chondrocytes erosion, and inflammation of joint tissue.
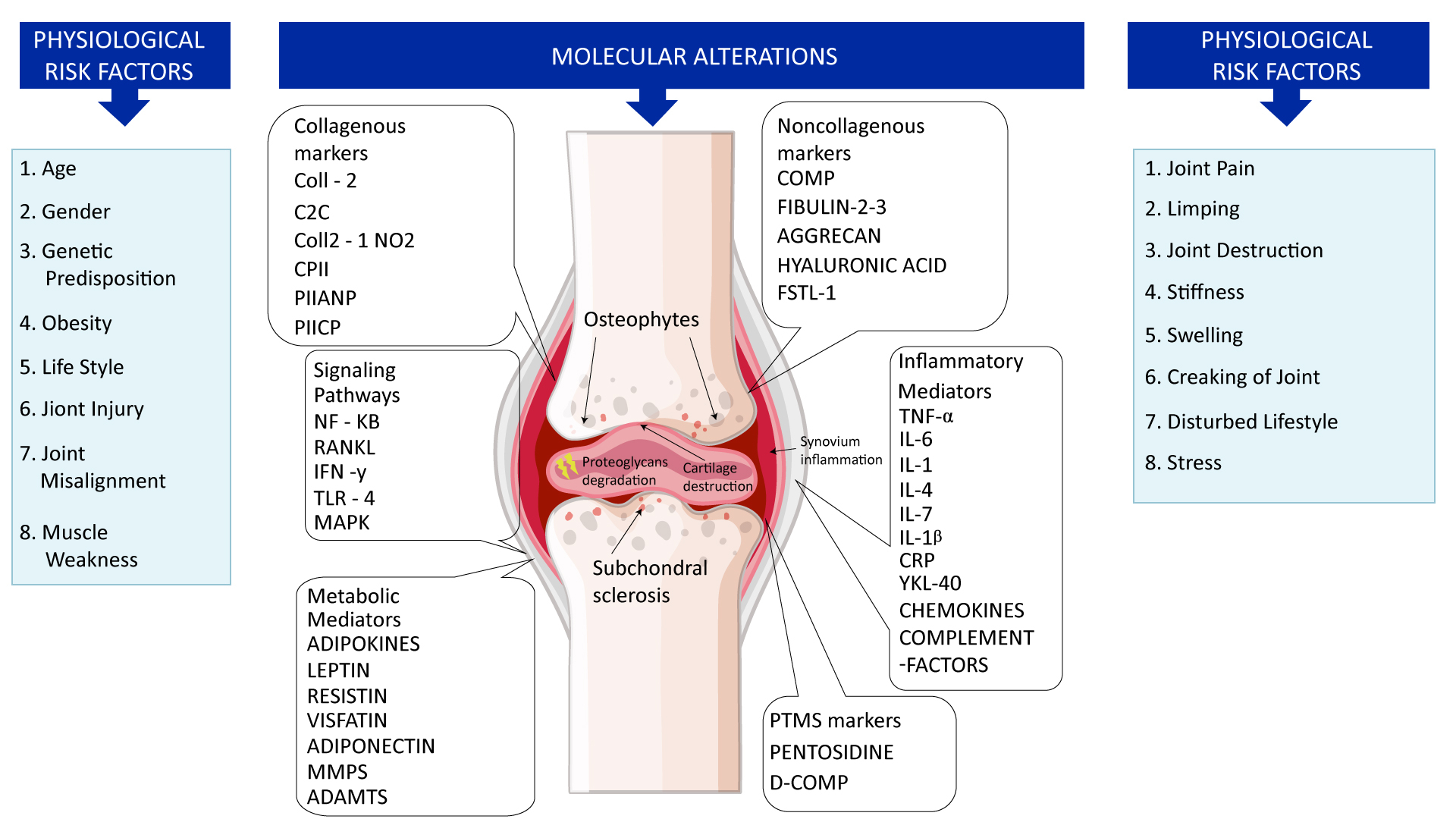
Figure 4: Physiological, molecular, and metabolic alterations are responsible for the cause of osteoarthritis.
The most commonly investigated biochemical markers include the following: ECM degradation—CTX-II, Coll2-1, C2C, C2M, Coll2-1NO2, cartilage oligomeric matrix protein (COMP), aggrecan epitopes (ARGS, TEGE, FFGV), fibulin-3 epitopes (Fib3-1, Fib3-2, Fib3-3), etc.; ECM synthesis—PIIANP, PIIBNP, CPII, CS846, and many others. Some of those cartilage metabolism biochemical markers have gained recognition in the field. For instance, urinary CTX-II (C-telopeptide fragments of type II collagen) is one of the better-known OA biochemical markers that has achieved a superior predictive profile compared to others. Both urine and SF CTX-II were associated with radiographic severity, while urine CTX-II was associated with pain in patients with early OA.
In the age of personalized and genomic medicine, biomarkers will continue to gain importance. OA biomarkers will become especially important due to the high prevalence and cost of OA. OA biomarker research has already helped deepen the understanding of the pathologic changes in OA. The ideal biomarker would be one that could be collected non-invasively, be predictive of the outcome of the disease, and provide potential therapeutic targets. The current markers all have advantages and disadvantages, as do each fluid studied. Blood and urine are easily accessible; however, changes in the synovial fluid can be detected earlier (Munjal et al., 2019).
|
Table 2: Molecular biomarkers and their role in pathophysiological function of osteoarthritis. | ||
|
Type |
Molecule name |
Function |
|
Serum protein (11-17 also found in synovia) |
Il-21, Il-17A, IFN‑γ |
Serum cytokines, B-cell proliferation, and differentiation |
|
Serum protein |
Il-4/ Il-4R |
Serum cytokine |
|
Fibulin-3 fragments |
Inhibition of chondrocyte differentiation and suppresses angiogenesis | |
|
arNOX |
ROS generation | |
|
ColX |
ECM maintenance during times of chondrocyte hypertrophy | |
|
COMP |
Likely involved in cartilage stabilization | |
|
miRNA-136 |
Multifactorial | |
|
miRNA-98 |
Related to immune system | |
|
miRNA 140-3p, 33b-3p, 671-3p, etc. |
Involved in many metabolic pathways including: Wnt, ErbB, | |
|
miR-675-5p, miR-126-5p, miR- 155-5p, etc. |
Many including apoptosis and cytokine expression | |
|
Urine ions |
Ca2+ and Zn2+ |
Tissue preservation, bone formation, signaling |
|
Urine metabolite |
CTX-II |
Associated with MMP activity |
|
Synovial protein |
Il-6 |
Osteoblast/osteoclast differentiation |
|
HIF |
Chondrocyte survival during hypoxia | |
|
Il-1B, TNF, and MMP |
Markers of OA | |
|
Lubricin |
Protects chondrocytes | |
|
TNF-α |
Signaling cell death | |
|
Synovial Peptide |
Bradykinin |
Vasodilation |
|
Synovial miRNA |
miRNA 132, 16, 146, etc. |
Multifactorial |
|
miRNA 181d-3p, 3904-3p, 155-3p, etc. |
Multifactorial, possibly involved in estrogen signaling | |
Currently, there are no reliable, quantifiable, and easily measured biochemical markers capable of providing an earlier diagnosis of OA, informing the prognosis of OA disease, and monitoring responses to emerging therapeutic modalities. The evaluation of structural changes in articular damage via imaging biomarkers [as determined by radiograph or magnetic resonance imaging (MRI)] is the most frequently used in clinical trials to evaluate subject eligibility and/or efficacy of the intervention, supporting decision making in OA drug development by ascertaining treatment effects on the joint structure. The European League against Rheumatism (EULAR) has formulated guidelines for imaging applications for the clinical management of OA in peripheral joints. The complex evaluation of biochemical markers of extracellular matrix (ECM) turnover, low-grade inflammation, and other biological processes may lead to a more specific evaluation of the catabolic and inflammatory aspects of OA. However, most of the studies with biochemical markers have focused on the late stages of the disease in humans or animal models. Studies in the early stages of OA are rare due to the lack of biochemical markers that permit the detection of early OA stages (Bernotiene et al., 2020).
Biomarkers in Gout
According to data reported in different studies, the global incidence rate is between 0.6-2.9/1000 person-years, and the prevalence rate is between 0.68% and 3.90% in adults. The incidence and prevalence of gout increase with age, and it is more common in men than in women. Xueshan Bai et al. found that serum CA72-4 levels are elevated in patients with frequent gout attacks and can be used as a predictor of gout attacks. However, the biomarkers related to gout inflammation are still unknown, limiting the prediction of gout flare and making atypical gout misdiagnosed or delayed diagnosis. The basic research on the pathogenesis of gout and clinical diagnosis and treatment are still in continuous progress and exploration (Qiu et al., 2022).
Emerging Biomarkers in Rheumatology
Major advances have been made in treating patients with rheumatic disease, but the choice of treatment strategy generally relies on a ‘trial-and-error approach’ because of a lack of biomarkers. Several studies are being conducted to identify the potential novel biomarkers. A ubiquitous intracellular chaperone protein 14-3-3η is expressed extracellularly in rheumatoid arthritis. 14-3-3η protein represents a novel biomarker for the detection of RA. It plays an important role in stimulating tumor necrosis factor-alpha, metalloproteinases, and other inflammatory mediators critical to joint erosion. This demonstrates its prognostic value with aims for treatment (Abdelhafiz et al., 2021). Research into the POS0562 autoantibodies to joint proteins has demonstrated its potential as a novel biomarker for diagnosing untreated early rheumatoid arthritis (Lönnblom et al., 2022).
Various studies have evaluated circPTPN22 to be associated with the classification of the RA process through ceRNA mechanisms. In one such study, circPTPN22 levels in RA PBMCs were correlated with RA‑IgG, RA‑IgM, RA‑IgA, anti‑cyclic citrullinated peptide (anti‑CCP), rheumatoid factor, and C reactive protein levels. A total of four putative microRNAs (miRNAs or miRs), namely, hsa‑miR‑3074‑5p, hsa‑miR‑373‑3p, hsa‑miR‑766‑3p, and hsa‑miR‑34c‑5p, were screened to be sponged by circPTPN22 via bioinformatics analysis and then experimentally verified to be upregulated in RA PBMCs compared with controls. The study suggested that circPTPN22 might be a novel biomarker for the diagnosis of RA and participate in RA pathogenesis through a sponge mechanism (Jiang et al., 2021).
Systemic inflammation response index (SIRI) and platelet/lymphocyte ratio (PLR) are positively correlated with disease activity in RA. SIRI has good accuracy in differentiating RA-ILD from RA patients without ILD. SIRI is generally higher in RA patients with tumor and could easily differentiate them from RA patients without tumor. Therefore, SIRI could be evaluated as a novel, noninvasive, and suitable biomarker for assisting in the diagnosis process and demonstrating the disease activity of RA, as well as predicting RA-ILD and tumor development of RA patients (Xu et al., 2022).
Circulating miR-124-3p and miR-377-3p may constitute promising biomarkers for the diagnosis of SLE. Researchers have noted the significant upregulation of miR-124-3p and miR-377-3p in Peripheral blood mononuclear cells and serum in SLE patients. The combined diagnostic efficiency of miR-124-3p and miR-377-3p is possibly higher than that of miR-124-3p or miR-377-3p alone. Expression of miR-124-3p in plasma was also associated with the various clinicopathological parameters such as C1q and C3. Certain Binary logistic regression analyses revealed that the expression of miR-377-3p was an independent predictor for SLE, and the plasma miR-124-3p was independently associated with the remission rate of SLE (Yan et al., 2022).
Furthermore, a strong association between Serum Uric Acid (UA) levels and the severity and progression of UIP in RA patients with ILD relative to RA patients without ILD has been reported. The increased serum UA level in patients with RA-ILD was correlated with HRCT-UIP score and RF. This suggested that the circulating UA concentration may be a potential biomarker for identifying ILD, especially UIP pattern, and evaluating the progression and severity of ILD in patients with RA (Wang et al., 2022).
Roles of lncRNAs are also being investigated as emerging novel biomarkers and therapeutic targets in SLE and RA. In SLE, lncRNAs such as GAS5, NEAT1, TUG1, linc0949, and linc0597 are dysregulated and may serve as emerging novel biomarkers and therapeutic targets. In RA, many validated lncRNAs, such as HOTAIR, GAS5, and HIX003209, have been identified as promising novel biomarkers for both diagnosis and treatment (Wu et al., 2021).
Challenges in Biomarker Developments in Rheumatology
A number of reasons can be put forward as to why personalized medicine is taking a long time to materialize in rheumatology. First, the heterogeneous and multifactorial nature of immune-mediated rheumatic diseases, with complex pathogeneses, makes it unlikely that a single marker of a given pathway will discriminate response of several different DMARDs with contrasting modes of action. Second, a considerable amount of effort is dedicated to identifying biomarkers in the blood, far from the key immunopathologic events happening in the synovial tissue, which may prove more informative. Third, it is not so commonly cited and relates to the subjective nature of a significant part of the tools used to assess treatment response, remission status, or disability. This applies both to the patient (e.g., visual analog scale) and the physician (e.g., joint counts) and is, by definition, influenced by many other individual-related factors, such as personality, previous experience with a given drug, expectations, patient-doctor relationship, cultural context, comorbidities, etc. Indeed, this scarcity of hard outcomes contrasts with that seen, for instance, in oncology (e.g., death, tumor-free survival), where personalized treatment has long been a reality. To what extent is the current situation explained by this fact is unclear, but subjective measures are likely to play an important role in confounding study results, potentially leading to the loss of a weak, albeit unique signal.
Conclusion
As we move forward, we anticipate a future where biomarkers are fully integrated with clinical practice and therapeutic development in rheumatology. An important goal for biomarker research is to help inform and personalize the choice of treatment recommended to patients to enhance the clinical benefit. Despite the extensive and expensive search for biomarkers in the past decades, very little has changed to personalize RA treatment. This research area uniquely demands insight and knowledge across the spectrum of science, from genotype to phenotype and laboratory to epidemiological methods. Therefore, identifying novel biomarkers with a clinical utility remains a major topic of interest.
Some of the promising biomarker combinations include a change in immunological parameters like cell kinetics with the appearance of autoantibodies, specific cytokine changes, and other markers of disease susceptibility. Others include anti-CCP testing, anti-CarP antibody testing, MBDA score, and anti-MCV. The targeted use of composite biomarkers in RA management paves the way for a transition from subjective measures of RA disease activity and treatment selection to more accountable, trackable, and reproducible objective measures.
Although biomarkers are very much needed, repeating the failed approach over and over again will unlikely get any closer. One of the major obstacles is controlling confounding, which can be partly addressed by testing drug adherence. Lack of statistical power is another factor that has hampered the reproducibility of biomarkers which can be addressed by increased funding for establishing real-world datasets and collecting biological samples as a part of the expected practice. As additional therapeutics and enabling technologies become available, stratified medicine based on biomarkers will increasingly take a leading role in the practice of medicine. Therefore, targeting specific biomarkers with the least side effects will bring hope to RA patients.
For more detailed analysis, visit: Rheumatoid Arthritis market
References
Abdelhafiz, D., Kilborn, S., & Bukhari, M. (2021). The role of 14-3-3 η as a biomarker in rheumatoid arthritis. Rheumatology and Immunology Research, 2(2), 87–90. https://doi.org/10.2478/RIR-2021-0012
Bernotiene, E., Bagdonas, E., Kirdaite, G., Bernotas, P., Kalvaityte, U., Uzieliene, I., Thudium, C. S., Hannula, H., Lorite, G. S., Dvir-Ginzberg, M., Guermazi, A., & Mobasheri, A. (2020). Emerging Technologies and Platforms for the Immunodetection of Multiple Biochemical Markers in Osteoarthritis Research and Therapy. Frontiers in Medicine, 7, 622. https://doi.org/10.3389/FMED.2020.572977/BIBTEX
Jiang, Z., Zhong, Z., Miao, Q., Zhang, Y., Ni, B., Zhang, M., & Tang, J. (2021). circPTPN22 as a novel biomarker and ceRNA in peripheral blood mononuclear cells of rheumatoid arthritis. Molecular Medicine Reports, 24(2), 1–11. https://doi.org/10.3892/MMR.2021.12256/HTML
Liu, S., Guo, Y., Lu, L., Lu, J., Ke, M., Xu, T., Lu, Y., Chen, W., Wang, J., Kong, D., Shen, Q., Zhu, Y., Tan, W. F., Ji, W., & Zhou, W. (2020). Fibrinogen-Like Protein 1 Is a Novel Biomarker for Predicting Disease Activity and Prognosis of Rheumatoid Arthritis. Frontiers in Immunology, 11, 2281. https://doi.org/10.3389/FIMMU.2020.579228/BIBTEX
Lönnblom, E., Agelii, M. L., Sareila, O., Hafström, I., Andersson, M., Cheng, L., Bergström, G., Ekwall, A. K. H., Rudin, A., Kastbom, A., Sjowall, C., Xu, B., Jacobsson, L. T. H., Viljanen, J., Kihlberg, J., Gjertsson, I., & Holmdahl, R. (2022). POS0562 AUTOANTIBODIES TO JOINT PROTEINS AS NOVEL BIOMARKERS FOR THE DIAGNOSIS OF UNTREATED EARLY RHEUMATOID ARTHRITIS. Annals of the Rheumatic Diseases, 81(Suppl 1), 546–546. https://doi.org/10.1136/ANNRHEUMDIS-2022-EULAR.3698
Mun, S., Lee, J., Park, M., Shin, J., Lim, M. K., & Kang, H. G. (2021). Serum biomarker panel for the diagnosis of rheumatoid arthritis. Arthritis Research and Therapy, 23(1), 1–10. https://doi.org/10.1186/S13075-020-02405-7/FIGURES/6
Munjal, A., Bapat, S., Hubbard, D., Hunter, M., Kolhe, R., & Fulzele, S. (2019). Advances in Molecular biomarker for early diagnosis of Osteoarthritis. Biomolecular Concepts, 10(1), 111–119. https://doi.org/10.1515/BMC-2019-0014/XML
Puentes-Osorio, Y., Amariles, P., Calleja, M. Á., Merino, V., Díaz-Coronado, J. C., & Taborda, D. (2021). Potential clinical biomarkers in rheumatoid arthritis with an omic approach. Autoimmunity Highlights 2021 12:1, 12(1), 1–21. https://doi.org/10.1186/S13317-021-00152-6
Qiu, K., Zeng, T., Liao, Y., Min, J., Zhang, N., Peng, M., Kong, W., & Chen, L. L. (2022). Identification of Inflammation-Related Biomarker Pro-ADM for Male Patients With Gout by Comprehensive Analysis. Frontiers in Immunology, 12, 5973. https://doi.org/10.3389/FIMMU.2021.798719/BIBTEX
Robinson, W. H., & Mao, R. (2016). Biomarkers to guide clinical therapeutics in rheumatology? Current Opinion in Rheumatology, 28(2), 168. https://doi.org/10.1097/BOR.0000000000000250
Saas, P., Toussirot, E., & Bogunia-Kubik, K. (2022). Editorial: Recent Advances in Potential Biomarkers for Rheumatic Diseases and in Cell-Based Therapies in the Management of Inflammatory Rheumatic Diseases. Frontiers in Immunology, 12, 5888. https://doi.org/10.3389/FIMMU.2021.836119/BIBTEX
Sturgeon, J. A., Finan, P. H., & Zautra, A. J. (2016). Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways. Nature Reviews Rheumatology 2016 12:9, 12(9), 532–542. https://doi.org/10.1038/nrrheum.2016.112
UK Health Security Agency. (2019). Why are musculoskeletal conditions the biggest contributor to morbidity? - UK Health Security Agency. https://ukhsa.blog.gov.uk/2019/03/11/why-are-musculoskeletal-conditions-the-biggest-contributor-to-morbidity/
van der Heijde, D., Daikh, D. I., Betteridge, N., Burmester, G. R., Hassett, A. L., Matteson, E. L., van Vollenhoven, R., & Lakhanpal, S. (2018). Common Language Description of the Term Rheumatic and Musculoskeletal Diseases (RMDs) for Use in Communication With the Lay Public, Healthcare Providers, and Other Stakeholders Endorsed by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR). Arthritis and Rheumatology, 70(6), 826–831. https://doi.org/10.1002/ART.40448/ABSTRACT
Wang, Z., Wang, W., Xiang, T., Gong, B., & Xie, J. (2022). Serum Uric Acid as a Diagnostic Biomarker for Rheumatoid Arthritis–Associated Interstitial Lung Disease. Inflammation, 1–15. https://doi.org/10.1007/S10753-022-01661-W/FIGURES/5
World Health Organization. (2021). Musculoskeletal conditions. https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions
Wu, H., Chen, S., Li, A., Shen, K., Wang, S., Wang, S., Wu, P., Luo, W., & Pan, Q. (2021). LncRNA Expression Profiles in Systemic Lupus Erythematosus and Rheumatoid Arthritis: Emerging Biomarkers and Therapeutic Targets. Frontiers in Immunology, 12, 5583. https://doi.org/10.3389/FIMMU.2021.792884/BIBTEX
Xu, Y., He, H., Zang, Y., Yu, Z., Hu, H., Cui, J., Wang, W., Gao, Y., Wei, H., & Wang, Z. (2022). Systemic inflammation response index (SIRI) as a novel biomarker in patients with rheumatoid arthritis: a multi-center retrospective study. Clinical Rheumatology 2022, 1–12. https://doi.org/10.1007/S10067-022-06122-1
Yan, L., Jiang, L., Wang, B., Hu, Q., Deng, S., Huang, J., Sun, X., Zhang, Y., Feng, L., & Chen, W. (2022). Novel microRNA biomarkers of systemic lupus erythematosus in plasma: miR-124-3p and miR-377-3p. Clinical Biochemistry. https://doi.org/10.1016/J.CLINBIOCHEM.2022.05.004
Executive Summary
Biomarker studies have added value to real-world data collections in inflammatory arthritis, and some of them have the potential to be used in routine clinical practice.